She began with a session overview and introduction, emphasizing that in kidney and bladder cancer, treatments are improving though the incidence is rising. However, we know that outcomes are not equal. For example, women with bladder cancer do more poorly as do older adults. Further, African American patients may do worse while clinical trial enrollees are overwhelmingly white. While the session seeks to deal with many of these issues, there are others that are important, but beyond the scope of what will be addressed in this session including differences due to rurality, financial and socioeconomic barriers, and the country of residence/treatment.
She began by highlighting the importance of equity in trial enrollment highlighting both that patients who are enrolled in clinical trials do better themselves than those who are not on trials and that trials contribute information regarding both the efficacy and safety of medications that will be used in the care of patients going forward (after approval).
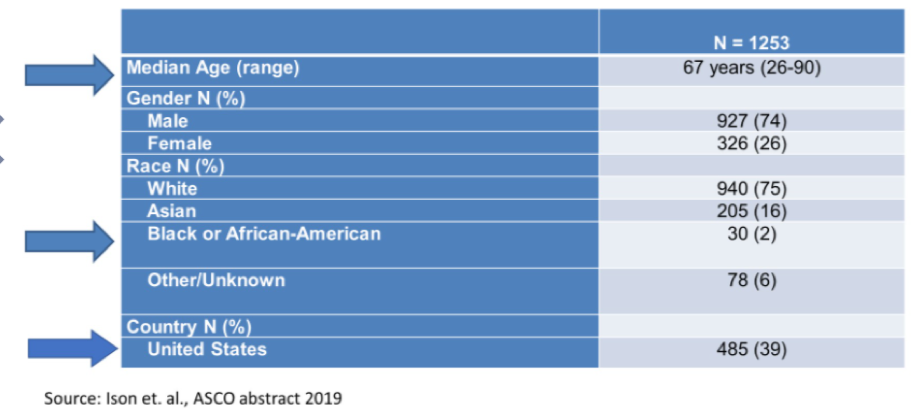
She highlighted the example of seven trials leading to the registration of immunotherapeutic agents in bladder cancer. The median age of the 1253 included patients was 67 years (range 26-90 years), 74% were male, 75% were white and only 2% were Black or African American. Further, Americans accounted for 39% of patients on these trials, reflecting the international nature of many of these studies.
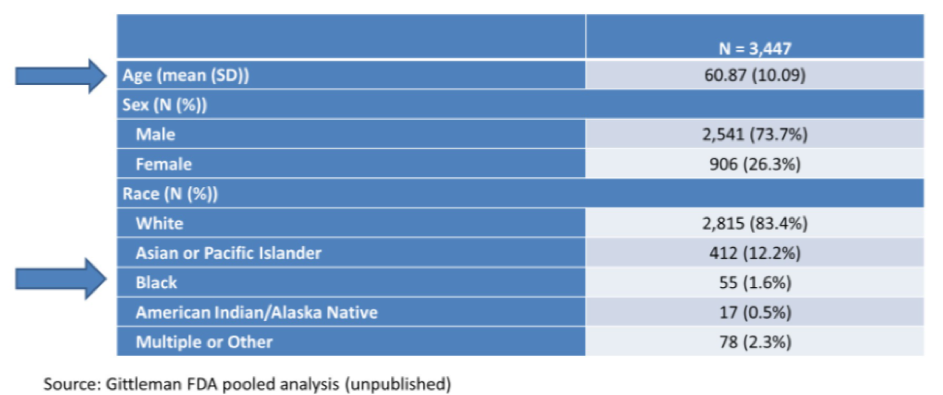
A generally similar pattern is seen among the 3447 patients enrolled on the four recent first-line trials of combination immunotherapy and tyrosine kinase inhibitor in kidney cancer: the mean age was 60.9 years and 74% were men. Racial disparities were even more profound among these trials with enrolled patients being 83.4% white and only 1.6% being Black.
Each of these examples highlights that clinical trial populations are younger than the population of cancer patients and heavily over-represented by White individuals.
She further emphasized that these presented data coming from submissions to the FDA. However, many of the studies included in the aggregate summaries presented above do not include a breakdown of enrollment by race in the published manuscripts. Thus, efforts to more transparently provide the racial composition of trial populations are both sorely needed and underway.
Dr. Weinstock then discussed concrete steps that may be taken to improve the enrollment of under-represented groups on clinical trials. These steps are adapted from a SWOG field guide.
- Involving stakeholders on trial design who represent the needs and interests of under-represented populations
- Engagement with diversity, equity, and inclusion experts, community advocates, and advocacy groups
- Plan and forecast enrollment to reflect the demographic trends for the disease of interest
- Proactively review the protocol to identify ways to make it easier for patients to enroll and remain on the study
- Engage in early outreach and educational efforts
- Plan accrual to account for diversity of enrollment between sites
- Monitor accrual in real-time, with diversity a key metric.
Dr. Weinstock then considered the reality of international trials, and patients treated outside the United States, is used to inform decisions for FDA approval. Regulations explicitly state that the use of “foreign data may be acceptable” but that the population included in the study “should reflect one found in the US” in terms of baseline characteristics and prior treatments. Further, patients from racial and ethnic minorities should be included to “recruit a diverse study population representative of the US”. However, this is clearly a standard that most trials, including those conducted entirely within the US, are unable to reach.
She further highlighted a number of ongoing projects within the FDA Oncology Center of Excellence including Project Equity and Project Silver. Project Equity is focused on developing policy and guidance to increase enrollment in clinical trials, on generating evidence through the lifestyle of a drug, fostering research collaborations, and integrating diverse perspectives in regulatory activities. In contrast, Project Silver has its key objective to improve the evidence base for treating older adults through a combination of regulatory policy, advocacy and outreach, global engagement, and research & publications. Other FDA guidance has focused on the inclusion of patients with HIV, Hepatitis B and C viruses, those with organ dysfunction or concurrent malignancies, and those with brain metastases. Additionally, there is work considering the minimum age considerations in pediatric populations.
In conclusion, she emphasized that COVID-19 has introduced some novel approaches to facilitate flexibility in trial conduct including remote monitoring and visits. While these may help many, the effects may not be consistent and may disproportionately disenfranchise those who are already marginalized and don’t have access to or a facility with technology.
Presented by: Chana Weinstock, MD, Genitourinary Oncology Team Leader at the United States Federal Drug Administration (FDA), Attending Oncologist Baltimore VA Medical Center GU Oncology Clinic
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


