KEYNOTE-045 is a randomized, multisite, open-label, phase 3 trial. Patients with histologically or cytologically confirmed urothelial carcinoma, progression after platinum-containing chemo, ECOG performance status 0-2, measurable disease per RECIST v1.1, and ≤2 prior lines of systemic therapy were eligible for enrolment. Patients were randomly assigned 1:1 to receive pembrolizumab 200 mg Q3W or investigator’s choice of paclitaxel 175 mg/m2 Q3W, docetaxel 75 mg/m2 Q3W, or vinflunine 320 mg/m2 Q3W. The trial design for KEYNOTE-045 is as follows:
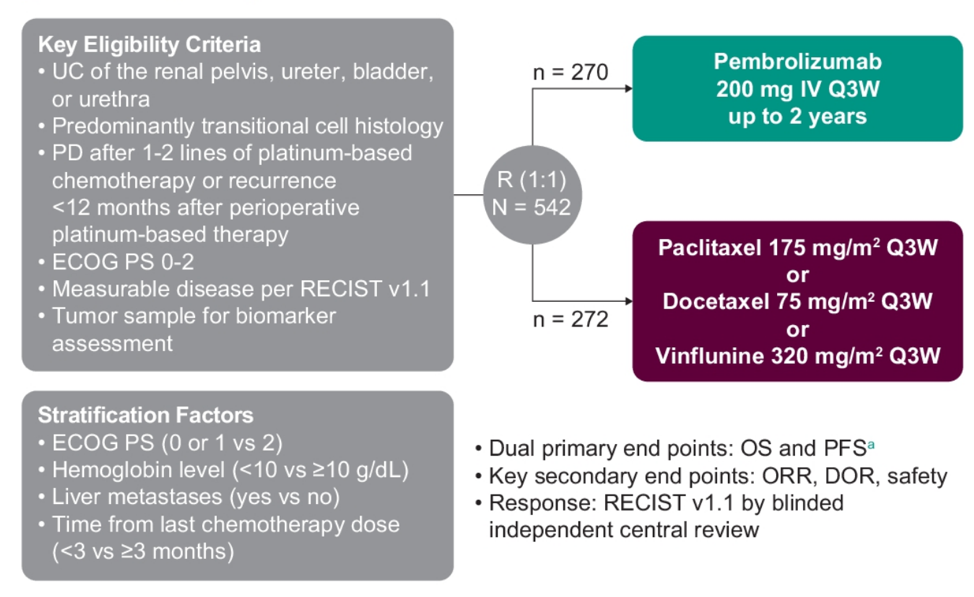
Primary endpoints for the trial are PFS (RECIST v1.1, blinded central review) and OS. Overall response rate and duration of response were key secondary endpoints.
As of October 1, 2020, among 542 enrolled patients, the median time from randomization to data cutoff was 62.9 months (range 58.6-70.9). Among patients in the pembrolizumab arm, 9.4% of patients completed two years of therapy compared to 0% of patients in the chemotherapy arm. The median OS was longer for pembrolizumab versus chemotherapy (10.1 versus 7.2 months; HR 0.71, 95% CI 0.59-0.86) overall
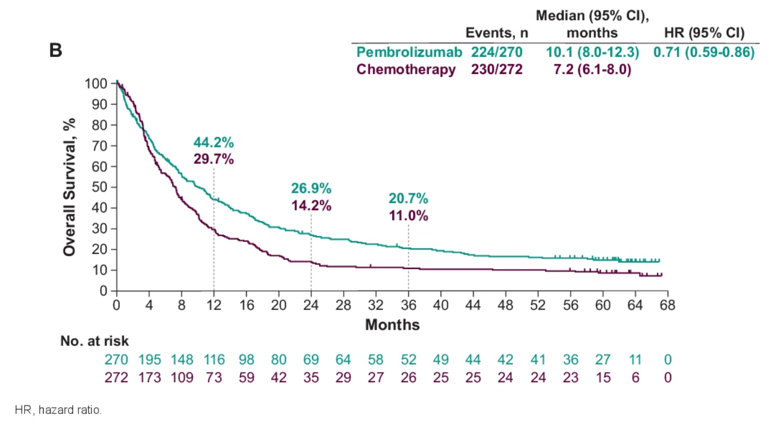
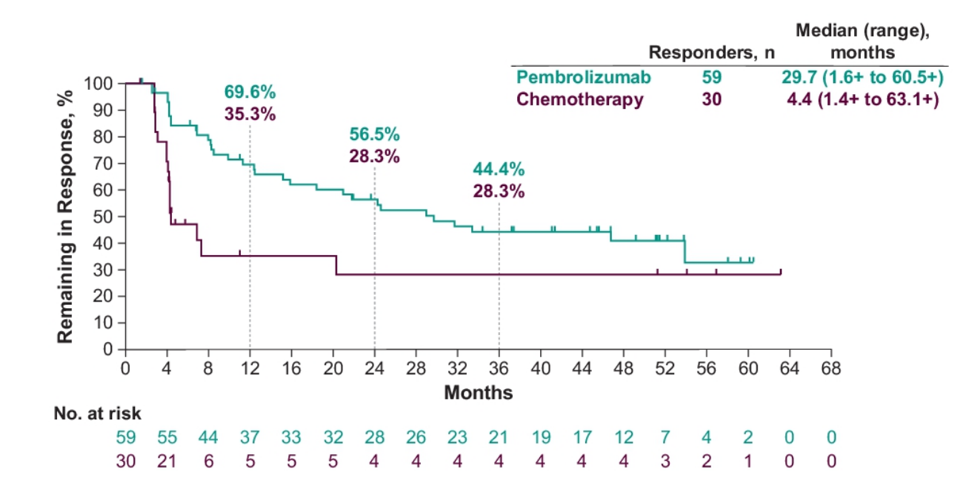
and in patients with CPS ≥10 (8.0 versus 4.9 months; HR 0.59, 95% CI 0.40-0.86). For patients with complete response or partial response, median OS was not reached and 16.4 (95% CI, 11.3-25.1) months in the pembrolizumab and chemotherapy arms, respectively. OS rates at 48 months were 16.7% for pembrolizumab and 10.1% for chemotherapy, and 60-month OS rates were 14.9% and 8.7%, respectively. Assessing subgroup analyses, the OS benefit with pembrolizumab versus chemotherapy continued regardless of age, ECOG performance status, prior therapy, liver metastases, baseline hemoglobin, time from last chemotherapy, histology, risk factors, and chemotherapy choice. The median duration of response for responders was longer for pembrolizumab versus chemotherapy (29.7 months [1.6+ to 60.5+] versus 4.4 months [1.4+ to 63.1+]), and a greater proportion of responses lasted ≥48 months (40.9% vs 28.3%) and ≥60 months (32.8% vs 28.3%):
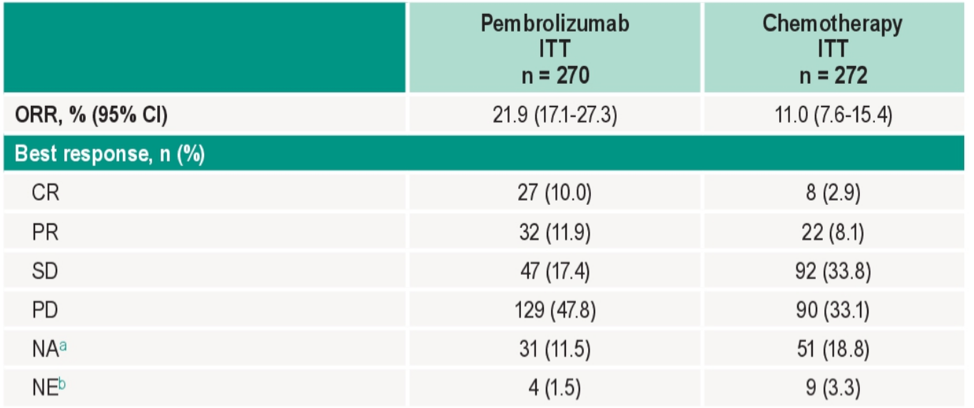
The objective response rate was higher for pembrolizumab versus chemotherapy (21.9% vs 11.0%; difference 10.8%)
and fewer patients were given pembrolizumab versus chemotherapy experienced a treatment-related adverse event of any grade (62.0% vs 90.6%) or grade ≥3 (16.9% vs 50.2%).
Dr. Bellmunt concluded this updated analysis of the KEYNOTE-045 trial with the following summary statements:
- After at least 5 years of follow-up, pembrolizumab monotherapy continued to show improved OS, objective response, and duration of response compared to investigator’s choice of chemotherapy in patients with advanced platinum-resistant or platinum-refractory urothelial carcinoma
- Safety was consistent with that of previous reports
- These data are consistent with the primary analysis and with the 2- and 3-year follow-up analyses that led to the approval of pembrolizumab for treatment of advanced platinum-resistant or platinum-refractory urothelial carcinoma irrespective of PD-L1 status
Clinical trial information: NCT02256436.
Presented by: Joaquim Bellmunt, MD, PhD, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
References:
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376(11):1015-1026.
- Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann Oncol. 2019 Jun 1;30(6):970-976.


