In 2020, the COVID-19 pandemic, COVID-19 vaccinations, and the death of George Floyd amplified concerns regarding health care inequities and delivery of care in the United States and globally. As such there has been a new call to action for diversity, equity and inclusion in medicine and cancer. Bladder and kidney cancer, and their clinical trials, are no exception.
Mr. Bangs poses the question, “What is equity for bladder and kidney cancer patients and their clinical trials?” Patients with cancer all seek the same thing: the best possible outcome, with the least possible treatment burden, which is a delicate balance that each patient will calibrate differently. Mr. Bangs notes that bladder and kidney cancer patients may believe that outcomes are insufficient on one or more dimensions:
- Incidence may be higher
- Treatments may not work as well, may not work as quickly, may not exist at all, and may not be locally available
- Treatments may have side effects, may cost too much, and may require too much time
- Outcomes may vary from patient to patient
At the patient level, equity would require that we can remove variability. However, Mr. Bangs notes that to achieve equity, we need to be able to understand variability in response and safety. That understanding must be coupled with increased diversity in clinical trial participation and supported by robust knowledge, extensive research and research funding, effective and widely available treatments, modest side effects, and good outcomes for each subpopulation. The question remains that if a patient is older, with comorbidities, African American, female, or lives in a rural setting, will the outcomes be the same as the patients on the clinical trials?
Clear barriers to equity are knowledge, research, and funding. Knowledge of kidney and bladder cancer is relatively immature compared to other cancers, leading to underfunding based on incidence and mortality rate. Research for kidney and bladder cancer has primarily focused on the rule, not the exception, with the larger subpopulations historically receiving the most funding, the most research emphasis, and the highest prioritization overall. Mr. Bangs suggests that by working from the bottom up rather than from the top down, we can recalibrate emphasis to other histologies, racial and ethnic subpopulations, and females. More research across a broader population is needed to elucidate the factors contributing to differences in incidence rates and outcomes within these subpopulations. As such, funding and emphasis are critical barriers and lack of funding for subpopulations cannot continue to have low priority or be characterized by something that we “just can’t afford.” Until this occurs, there can be no equity, according to Mr. Bangs. More traditional barriers cannot be overlooked, which include health literatures (includes being health system savvy), pre-existing conditions, trust, and logistical cost and time. Mr. Bangs notes that the data discussing logistical cost and time are far too sparse. For cancers such as kidney and bladder cancer that require multiple treatments and visits, logistical costs and time are huge barriers.
Critical factors to success in attaining diversity, equity and inclusion is buy-in by key stakeholders, including but not limited to the following:
- American Society of Clinical Oncology
- Food and Drug Administration
- Industry partners
- The National Cancer Institute
- The National Clinical Trials Network groups
- Professional oncology societies
- Institutions and care facilities
- Advocacy groups
- Payers
Leveraging a diversity, equity, and inclusion vision and guidance, as clinical trialists, we must institute a methodology to build representativeness into our trials from the ground up. Such a methodology with equity as a goal is not currently in the public domain and represents a significant barrier. At the core of instituting this methodology are four critical success factors: (i) resources (including funding and people), (ii) outreach, (iii) goal setting and management, and (iv) plans & countermeasures: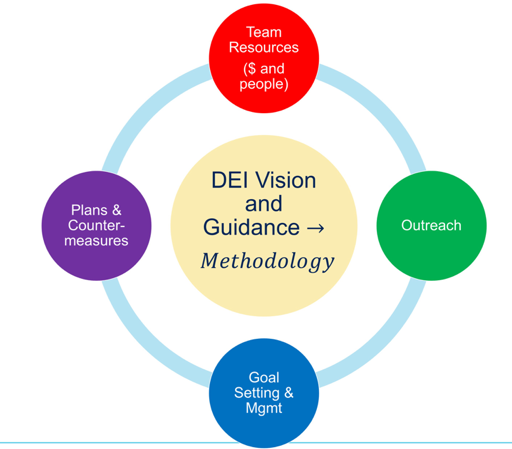
In more detail, the following is the methodology to improve diversity and representativeness in clinical trials across the lifecycle:
Mr. Bangs notes that the above methodology must be put into the context of each situation, for example not every clinical trial will have sufficient size to leverage it. Calibration will be critical. Mr. Bangs’ group at SWOG has also developed 30-45 minute training modules, as well as field guides to provide additional, hands-on guidance on the application of each strategy.
Mr. Bangs concluded by noting that in 2021 the need to eliminate disparities in cancer, particularly kidney and bladder cancer and their related clinical trials, is clearer than ever. He believes we are poised to make significant progress with a little recalibration and focus on continuous improvement. The patients we collectively serve, particularly older adults (including those that are ineligible for surgery or chemotherapy), African Americans, and females are all anxious for change and better possibilities for those that follow in their footsteps. We need to and can deliver equity to every patient, every day, everywhere.
Presented by: Rick Bangs, MBA, PMP, Cancer Research Advocacy Leadership, bladder cancer advocate, Bladder Cancer Advocacy Network


