(UroToday.com) Inducible T-cell co-stimulatory expression is induced on T-cells following T-cell receptor activation and previous studies have demonstrated that inducible T-cell co-stimulation is highly expressed in urothelial carcinoma across all stages of disease.
Feladilimab is a first-in-class humanized IgG4 mAb selected for its agonist activity through the inducible T-cell co-stimulatory receptor, with low/no T-cell depleting effects. It was previously shown that feladilimab induced IFNγ, increased PD-1/L1 expression, and enhanced antitumor activity in combination with PD-1 blockade non-clinically. INDUCE-1 is a first-in-human trial evaluating feladilimab as monotherapy and in combination with pembrolizumab. Expansion cohorts include tumor types, such as urothelial carcinoma, with high inducible T-cell co-stimulatory expression and immunotherapy-favorable features. At the 2021 ASCO annual program’s urothelial carcinoma oral abstract session, Dr. Arjun Balar and collaborators presented results of their study assessing preliminary efficacy, safety, and biomarker data of feladilimab ± pembrolizumab in INDUCE-1 urothelial carcinoma expansion cohorts.
Eligible patients had recurrent/metastatic urothelial carcinoma of the upper or lower urinary tract, ≤6 prior systemic therapy lines in the advanced setting, measurable disease, and no active autoimmune disease. Patients received 0.3 or 1 mg/kg feladilimab (monotherapy expansion cohort; anti-PD-1/L1–experienced patients) or 0.3 mg/kg feladilimab+ 200 mg pembrolizumab (combination expansion cohort; anti-PD-1/L1–naïve patients) every 3 weeks, up to 35 cycles until disease progression or unacceptable toxicity. Disease was assessed every 9 weeks through week 54, then every 12 weeks. Archival and/or fresh biopsy tumor tissue was collected for biomarker analyses and safety assessed. The trial design for INDUCE-1 is as follows: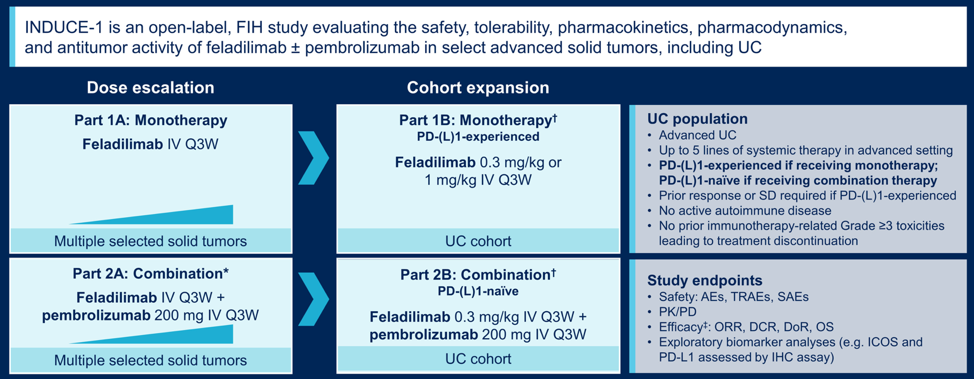
As of March 16, 2021, 14 anti-PD-1/L1–experienced and 32 anti-PD-1/L1–naïve patients were evaluable in the monotherapy and combination expansion cohorts, respectively. In the monotherapy expansion cohort, median age was 69 years (range: 40–82), 93% of patients were male, and 86% received ≥2 prior therapy lines in the metastatic setting. In the combination expansion cohort, median age was 70 years (range: 42–84), 75% of patients were male, and 72% received ≥1 prior therapy line in the metastatic setting. In the monotherapy expansion cohort, median duration of follow-up was 10.6 months (range: 1.1–22.8); overall response rate was 7% (1 partial response; 95% CI 0.2, 36.0) with a duration of response of 6.1 months. Disease control rate (response or stable disease for ≥9 weeks) was 21% (95% CI 4.7, 50.8), and median overall survival (OS) was 14.5 months (95% CI 3.0, NR), with 77% of patients alive at 6 mo. In the combination expansion cohort, median duration of follow-up was 9.6 months (range: 0.9–28.3); objective response rate was 22% (7 partial responses; 95% CI 9.3, 40.0) with a median duration of response of 8.3 months (range: 3.5–23.3+). Disease control rate was 63% (95% CI 43.7, 78.9), and median OS was 10.7 months (95% CI 5.2, 18.4), with 64% of patients alive at 6 months. 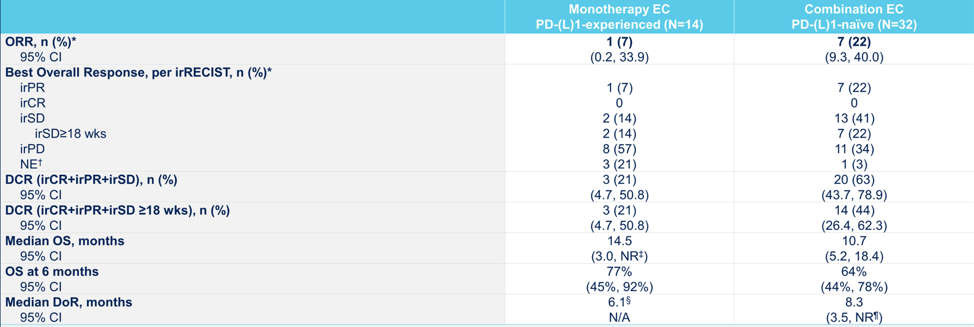
Best tumor response in PD-L1 positive subcohorts is as follows for the monotherapy and combination cohorts: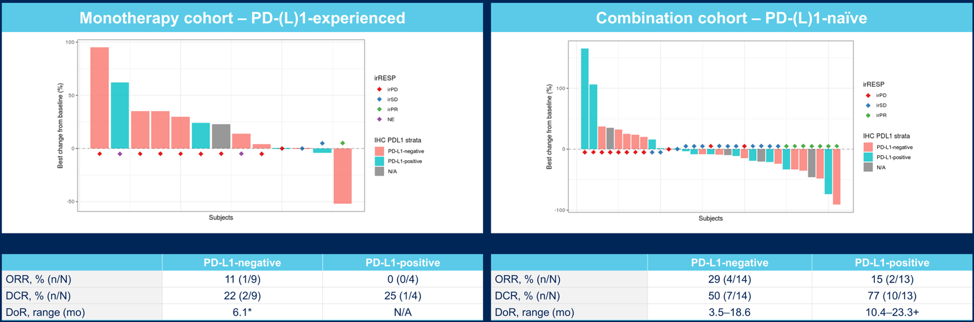
Best tumor response in inducible T-cell co-stimulatory-enriched subcohorts is as follows for the monotherapy and combination cohorts: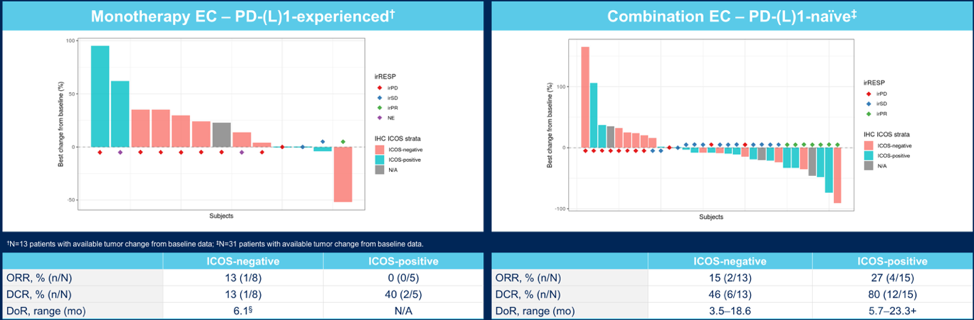
Grade ≥3 treatment-related adverse events were reported for 0% and 9% of patients in the monotherapy (n = 16) and combination (n = 44) safety populations, respectively. PD-L1 expression and inducible T-cell co-stimulatory-specific biomarkers are being evaluated, with promising trends observed in the enrichment of clinical activity in preliminary analyses.
Dr. Balar concluded his presentation of the INDUCE-1 trial with the following take-home messages:
- Feladilimab monotherapy and feladilimab in combination with pembrolizumab had a manageable safety profile in advanced urothelial carcinoma
- Immune-related adverse events were not formally evaluated, however rates of adverse events with a potential immune-mediated etiology were low
- Feladilimab demonstrated evidence of clinical activity as monotherapy in heavily pre-treated, PD-(L)-1-experienced advanced urothelial carcinoma and in combination with pembrolizumab in anti-PD-(L)-1-naïve advanced urothelial carcinoma
- Feladilimab is the first inducible T-cell co-stimulatory agonist with reported single-agent activity in anti-PD-(L)-1-experienced advanced urothelial carcinoma, supporting inducible T-cell co-stimulation as a therapeutic target. Durable responses were observed in the combination therapy cohorts
- The feladilimab monotherapy and combination with pembrolizumab data in urothelial carcinoma is under evaluation to assess the impact on the overall clinical development program
Clinical trial information: NCT02723955
Presented by: Arjun V. Balar, MD, Perlmutter Cancer Center at NYU Langone Health, New York, NY
Co-Authors: Victor Moreno, Eric Angevin, Hui Kong Gan, Maria Vieito, Antoine Italiano, Riccardo Danielli, Erminia Massarelli, Frans Opdam, Michael Jon Chisamore, Debra Rogan, Xiao Ji, Courtney Henry, Catherine Elizabeth Ellis, Marc S. Ballas, Axel Hoos, Francesco Ricci; START Madrid-FJD, University Hospital “Fundacion Jimenez Diaz”, Madrid, Spain; Gustave Roussy Institut de Cancérologie, Villejuif, France; Department of Medical Oncology, Austin Hospital, Melbourne, VIC, Australia; Vall d'Hebron Institute of Oncology (VHIO), Vall d'Hebron University Hospital (HUVH), Barcelona, Spain; Institut Bergonié, Bordeaux, France; University Hospital of Siena, Siena, Italy; City of Hope Cancer Center, Duarte, CA; The Netherlands Cancer Institute, Amsterdam, Netherlands; Merck & Co., Inc., Kenilworth, NJ; GlaxoSmithKline, Research Triangle Park, Durham, NC; GlaxoSmithKline, Collegeville, PA; Department of Drug Development and Innovation (D3i), Institut Curie, Paris, France
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting #ASCO21, June, 4-8, 2021


