The case presentation from Dr. Yu was a 62-year-old female who presented with gross hematuria and underwent cystoscopy, which showed several areas of raised erythema in the bladder. The patient ultimately underwent a biopsy that showed carcinoma in situ, which Dr. Lee notes is an important diagnosis to make early, as it is a precursor to invasive disease. This person would likely be a candidate for intravesical therapy, which Dr. Lee notes the gold standard is still BCG therapy. She also highlighted that approximately 2/3 of patients will respond to BCG, but up to half of those responders will eventually suffer disease relapse. Dr. Lee notes that it is important for these patients that respond to induction BCG to subsequently receive adequate maintenance therapy, as it has been shown in multiple studies and meta-analyses to decrease not only the risk of recurrence but also disease progression.
This patient subsequently underwent induction intravesical BCG therapy x 6 doses, which was followed by maintenance therapy. However, at the time of her six-month maintenance therapy/cystoscopy, she had a papillary bladder recurrence. At this point she underwent upper tract imaging (which was negative) and repeat TURBT now shows a T1 tumor with concomitant CIS:
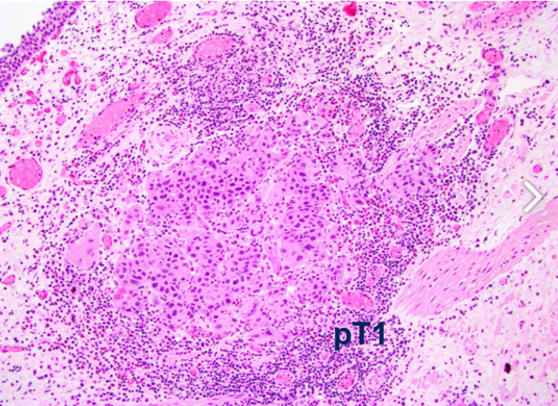
Dr. Hahn notes that this patient is now considered BCG-unresponsive, non-muscle-invasive bladder cancer, given the time frame from adequate BCG treatment to the pathologic findings. Dr. Yu then posed the following question to the panel: “What treatment option would you proceed with?”
- Intravesical chemotherapy
- Intravenous pembrolizumab
- Radical cystectomy
- Other
Dr. Lee notes that given her good performance status and rapid relapse, she would opt for upfront radical cystectomy. However, other options are now FDA-approved pembrolizumab, as well as intravesical chemotherapy with gemcitabine plus docetaxel. Dr. Lee also highlighted that the patient should undergo repeat TURBT unless the patient was going for upfront cystectomy at which point she would not perform repeat TURBT. Dr. Hahn further elaborated on the utilization of pembrolizumab, which is approved in this disease space, however, given the T1 component, perhaps this patient is not the optimal candidate for BCG-failure pembrolizumab. However, Dr. Hahn highlighted that this patient technically is eligible for the KEYNOTE-057 trial, with the caveat that the majority of patients in the trial were true CIS alone1.
The patient subsequently refuses radical cystectomy. Due to distance and necessary travel, she is also not willing to receive every three weeks pembrolizumab. She is willing to receive intravesical chemotherapy and the decision is made to proceed with intravesical gemcitabine and docetaxel. Dr. Lee notes that valrubicin would be an option here, given that it is FDA approved, but it is not commonly used in practice given the lack of long-term efficacy. Nadofaragene firadenovec is another option (although not yet FDA approved), which is attractive based on its favorable intravesical delivery schedule of every three-month dosing2.
The patient subsequently underwent intravesical chemotherapy, but thereafter surveillance cystoscopy performed at six months reveals invasive disease obscuring the bilateral ureteral orifices. Her creatinine clearance is found to be 38 mL/min and she has bilateral hydronephrosis on CT imaging, without significant adenopathy. TURBT confirms that this patient now has T2 disease. Dr. Yu poses the question: “Agree or disagree: bilateral percutaneous nephrostomy tubes are preferred over internal ureteral stenting.” Dr. Lee notes that patients are never excited about external drains and tubes, but it is the most efficient way to preserve kidney function and practically it can be difficult to place ureteral stents when the disease is obscuring the ureteral orifices. From a medical oncology perspective, Dr. Hahn notes that percutaneous nephrostomy tubes are more reliable and allow for avoiding delays in systemic therapy.
The patient is recommended to receive bilateral percutaneous nephrostomy tubes, and the patient wants to start treatment as soon as possible. Her ECOG performance status is 1, and she has no other major comorbidities. What options do you consider?
- Radical cystectomy
- Split-dose cisplatin-combination chemotherapy followed by cystectomy
- Carboplatin-combination chemotherapy followed by cystectomy
- Pembrolizumab followed by cystectomy
- Bladder preservation with cisplatin-based chemoradiation
- Bladder preservation with 5-FU-based chemoradiation
Dr. Vapiwala notes that patients do like the idea of bladder preservation, such as trimodality therapy. Given this patient’s hydronephrosis and need for percutaneous nephrostomy tubes for poor kidney function, perhaps trimodality therapy is not the optimal treatment option for this patient. However, it is important to take a personalized approach for these treatment options and discuss all therapies. Dr. Hahn notes that given her creatinine clearance, even split-dose treatment, her cisplatin eligibility is questionable. However, given that we are still looking for a curative approach, sometimes medical oncologists will push the creatinine clearance threshold to ~45 ml/min or 50 ml/min for cisplatin eligibility. Furthermore, there is provocative phase 2 data from the PURE-01 neoadjuvant pembrolizumab suggesting that pT0 rates of ~40% are possible after radical cystectomy3.
After bilateral percutaneous nephrostomy tubes are placed, the creatinine clearance improves to 61 mL/min and her ECOG performance status remains at 1. The patient states that she would prefer to undergo surgery, but she is willing to receive neoadjuvant chemotherapy if recommended. Dr. Yu then asked the panel “Agree or disagree: My preferred chemotherapy treatment regimen is cisplatin with gemcitabine.” Dr. Hahn notes that gemcitabine plus cisplatin is the preferred regimen, but states that dose-dense (or accelerated) MVAC is also an option for this patient.
The patient then undergoes four cycles of accelerated MVAC chemotherapy followed by radical cystectomy with an ileal conduit. She is found on pathology to have residual ypT2N0 disease. What course of action would you recommend next?
- Adjuvant carboplatin with gemcitabine
- Adjuvant taxane-combination chemotherapy regimen
- Adjuvant checkpoint inhibitor
- Observation
Dr. Lee notes that residual muscle-invasive bladder cancer after radical cystectomy is a worse prognosis when compared to patients that have <=pT1 or pT0 disease. Given that the disease is still organ fined on pathological staging, Dr. Lee states that she would probably opt for observation at this point in time. Dr. Hahn would also observe this patient based on the guidelines, however, there is recent data suggesting that adjuvant checkpoint inhibitor data may change our practice in the near future.
Dr. Yu then asked the panel “If instead, the post-operative pathology revealed residual ypT2R1N0 disease, would this change your recommended course of next action?” And “Agree or disagree: I would recommend adjuvant radiation therapy” Dr. Vapiwala states that in this setting the data is not clear regarding the efficacy of adjuvant radiotherapy. However, in subgroup analyses of published data, there is a suggestion that patients with positive surgical margins may derive a benefit from adjuvant radiotherapy.
The patient with ypT2N0 residual disease undergoes observation and six months later, surveillance CT imaging reveals liver metastases: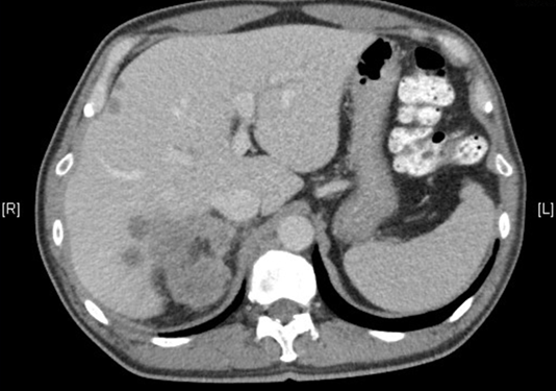
Her creatinine clearance has drifted back down to 48 ml/min and her ECOG performance status remains 1. Dr. Yu then asked the panel “Agree or disagree: I would perform PD-L1 immunohistochemistry on archival tissue to aid in treatment decision making.” Dr. Hahn notes that in his practice, he would likely not perform PD-L1 testing given that she now has visceral metastatic disease, which is a poor prognostic factor in the post-platinum setting. Additionally, it cannot be overstated that understanding a patient’s goals given the current clinical situation is important.
The patient undergoes PD-L1 testing with 22C3 antibody and the CPS score returns at <1. What course of action would you take next?
- Gemcitabine with cisplatin
- Gemcitabine with carboplatin
- Pembrolizumab
- Gemcitabine with carboplatin plus atezolizumab
Dr. Hahn states that he would likely consider giving pembrolizumab since the patient relapsed so soon after her platinum-based chemotherapy. Dr. Yu then posed the following question to the panel “Agree or disagree: if the patient relapsed 15 months, instead of six months after neoadjuvant chemotherapy and radical cystectomy, that would change my systemic treatment selection.” Dr. Hahn states that he would now give more thought to treating with chemotherapy, especially with a CPS score < 1.
A follow-up question from Dr. Yu is “Agree or disagree: I would obtain next-generation sequencing of the tumor at this time.” Dr. Hahn agrees that at this point he would obtain next-generation sequencing with the hope that it may bring into play additional therapies, for example, if there were an FGFR alteration. The patient undergoes Foundation Medicine next-generation sequencing which reveals a microsatellite stable tumor without hypermutation. There is no FGFR alteration and no other actionable genetic alterations. The patient has a response to pembrolizumab for 1 year before liver metastases being to grow and new lung and bone metastases are found on restaging imaging. What treatment would you proceed with next?
- Taxane chemotherapy
- Enfortumab vedotin
- Erdafitinib
- Sacituzumab govitecan
Dr. Hahn states that at this point he would proceed with enfortumab vedotin systemic therapy for two reasons 1) the presence of liver metastases, since enfortumab vedotin has shown activity in liver metastases (~33%) and 2) enfortumab vedotin has a survival benefit in this setting [4]. The patient responds to enfortumab vedotin for 9 months before developing increasing low back pain. ECOG performance status is now down to 3. After obtaining a spine MRI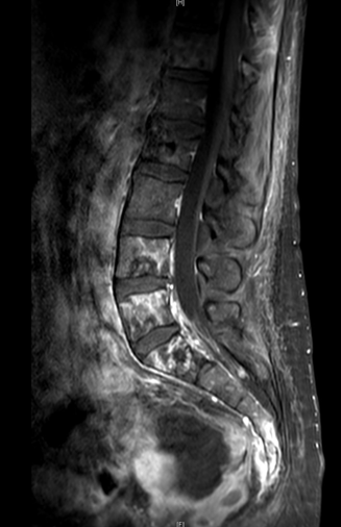
what will be your next course of action?
- Hospice
- Radiation therapy
- Surgical decompression
- Palliative chemotherapy and bisphosphonates
Dr. Vapiwala states that at this point in time she would make sure to once again discuss with the patient goals of care. Since the patient is in pain and this is oligometastatic bone disease, there would likely be a role for radiation therapy, specifically SBRT, to the metastatic disease site.
Key take-home points from this panel discussion are as follows:
- Pembrolizumab is FDA approved for BCG unresponsive non-muscle-invasive bladder cancer in patients who are not fit for or who refuse radical cystectomy
- For cisplatin-ineligible patients with muscle-invasive localized urothelial bladder cancer, radical cystectomy is the preferred treatment option without systemic therapy
- Hydronephrosis is a poor prognostic sign for patients undergoing bladder preservation with chemoradiation
- Residual muscle-invasive disease after radical cystectomy is a poor prognostic sign
- Pembrolizumab offers survival benefit for patients in the post-platinum metastatic setting (including <1 year from perioperative platinum)
- Pembrolizumab and atezolizumab are FDA approved for patients with metastatic disease who are ineligible for cisplatin but who have high PD-L1 tissue immunostaining and also for platinum-ineligible patients
- FGFR testing should be considered for patients with metastatic urothelial carcinoma to prepare potentially for the post-platinum setting where erdafitinib may be used for an FGFR2 or FGFR3 alteration
- Enfortumab vedotin offers survival benefit and is FDA approved for patients with metastatic urothelial carcinoma who have received prior platinum chemotherapy and PD-1/PD-L1 antibody therapy
- Sacituzumab govitecan is newly approved on the accelerated pathway for metastatic urothelial carcinoma who have received prior platinum chemotherapy and PD-1/PD-L1 antibody therapy
Medical Oncologist: Noah M. Hahn | Departments of Oncology and Urology, Johns Hopkins School of Medicine
Radiation Oncologist: Neha Vapiwala, University of Pennsylvania, Philadelphia, PA
Urologic Oncologist: Cheryl T. Lee, The Ohio State University, Columbus, OH
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting #ASCO21, June, 4-8, 2021
References:
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021 May 26;S1470-2045(21)00147-9.
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2020 Nov 27:S1470-2045(20)30540-4.
- Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol 2018 Dec 1;36(34):3353-3360.
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021 Mar 25;384(12):1125-1135.


