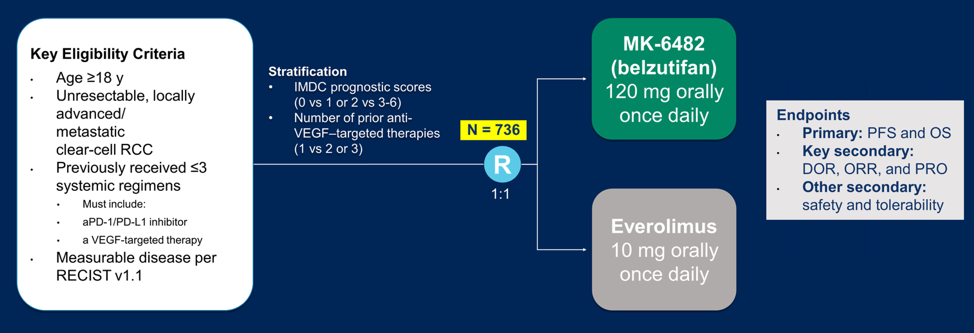
Dr. Albiges notes that understanding tumor biology defines our novel therapeutics available. HIF-2alpha blockade has been evaluated in the phase I/II setting, suggesting activity of belzutifan following first-line IO therapy. Among 55 patients (62% with >= 3 lines of prior therapy), 64% had a reduction in target lesions size, the objective response rate was 25%, disease control rate was 80%, median PFS was 14.5 months, however, there were no complete responses. With regards to the safety profile, this was distinct from VEGFR inhibitors in that 27% of patients had grade 3 anemia and 16% of patients had grade 3 hypoxia. There are ongoing HIF-2alpha studies in the salvage setting, including the single-agent phase 3 study (NCT04195750) randomizing patients to belzutifan versus everolimus (n=736):
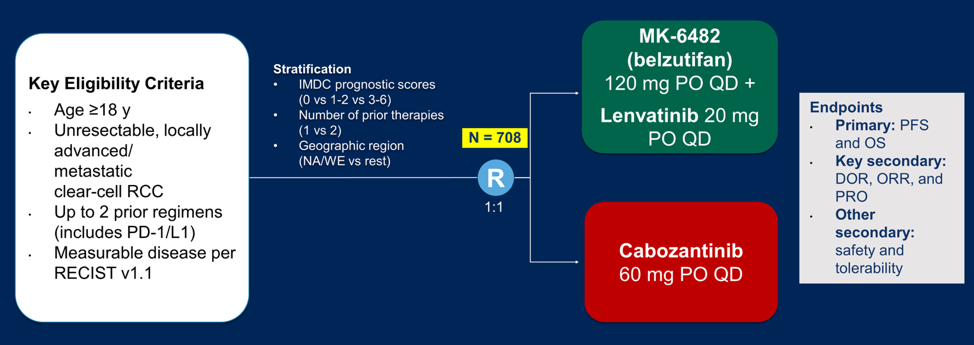
Based on the unique mechanism of action, HIF2-alpha inhibitors have also been evaluated in combination therapy. Presented by Dr. Brian Rini at GU ASCO 2019, PT2385 + nivolumab (n=50) demonstrated an objective response rate of 22%, disease control rate of 64%, and median PFS of 7.3 months. Presented by Dr. Toni Choueiri at GU ASCO 2021, belzutifan + cabozantinib (n=52) demonstrated an objective response rate of 22%, disease control rate of 90%, and median PFS of 16.8 months. Given this encouraging data, belzutifan combination treatment is being tested in a phase 3 trial of belzutifan + lenvatinib versus cabozantinib:
According to Dr. Albiges, there are several open questions/challenges with regards to HIF-2alpha inhibition:
- Single-agent activity is still under evaluation
- Biological and clinical rationale demonstrating potential synergy in combination with PD-1/immune checkpoint blockade and in combination with VEGFR-TKI
- Where do we fit HIF2-alpha inhibition in the treatment landscape? Early/upfront rescue?
- Understanding the mechanism of resistance
The third point to understanding tumor biology and developing novel therapeutics includes enhancing immune response. This involves potential new immune checkpoint blockade agents, cell therapy, as well as perhaps epigenetic immune modulators and personalized vaccines. Potential candidates to modulate the immune response are as follows:
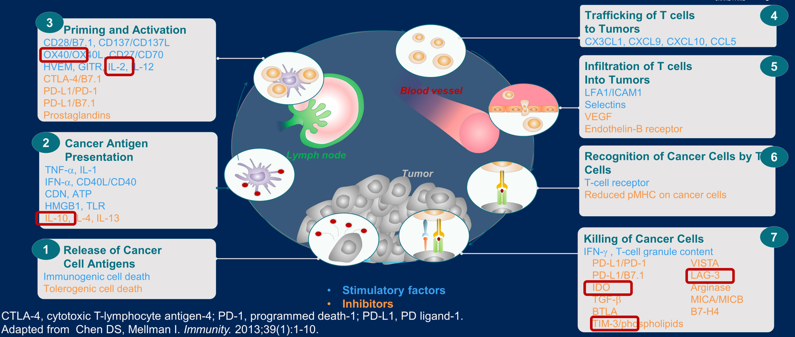
Bempegaldesleukin is a PEGylated IL-2 acting as a CD122-preferential IL-2 pathway agonist and is being tested in the first-line setting in combination with nivolumab versus investigators choice of sunitinib or cabozantinib. The co-primary endpoints for this trial are objective response rate and overall survival.
Other new immune checkpoint blockade targets include:
- TIGIT: tiragolumab, which has been granted FDA breakthrough designation in combination with atezolizumab for first-line treatment of metastatic NSCLC whose tumors have high PD-L1 expression
- HHLA2: overexpressed in RCC and does not overlap with PD-1. Inhibition of KIR3DL3 allows for engagement of an antitumor immune response
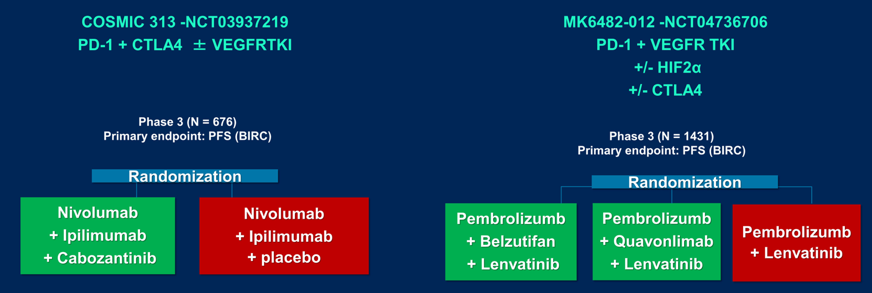
Second, is assessing for a treatment tailored approach, such as aiming de-escalation of treatment for appropriate patients, which is being evaluated in trials such as the RCC-TITAN phase 2 trial of tailored nivolumab, and the PDIGREE trial tailoring treatment after induction of nivolumab + ipilimumab. Third, is using biomarkers to select upfront combination therapies, of which such trials have been proven feasible based on the BIONIKK trial first presented at ESMO 2020. Fourth, is considering the gut microbiota as a therapeutic approach, given that gut bacteria drives primary resistance to immunotherapy in RCC. Interventional studies are ongoing to modulate the gut microbiome in patients with mRCC receiving checkpoint inhibitors in order to assess for clinical impact. Fifth is the consideration of adjuvant therapy impacting the algorithm of treatment. Being presented later at this meeting by Dr. Choueiri is the KEYNOTE-564 trial, which showed a DFS benefit for adjuvant pembrolizumab following radical nephrectomy. However, Dr. Albiges notes that we need to understand whether a DFS benefit will translate to an OS benefit, whether there are considerations for patient selection, and what the impact is on treatment sequence given that patients that recur after adjuvant treatment will already be post-checkpoint inhibitor therapy.
Dr. Albiges concluded her presentation with the following take-home messages:
- There are new agents, such as HIF-2alpha inhibition, that are under evaluation
- New combinations are being assessed and perhaps there will be a role for triplet therapy
- Currently, there are insufficient biomarker driven trials
- There is potentially a new algorithm requiring us to focus on a PD-1 resistance strategy
Presented by: Laurence Albiges, MD, PhD | Department of Cancer Medicine, Gustave Roussy Cancer Campus, University of Paris Sud
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


