The authors enrolled patients with IMDC intermediate and poor-risk advanced clear cell RCC between October 2016 and December 2018. Patients began treatment with nivolumab 240 mg every 2 weeks induction. Patients with early significant progressive disease (week 8) or non-responders at week 16 received 2-4 nivolumab + ipilimumab “boost” cycles as salvage therapy. Patients who initially responded (partial or complete response) to nivolumab monotherapy continued with maintenance but could receive nivolumab + ipilimumab for later progressive disease.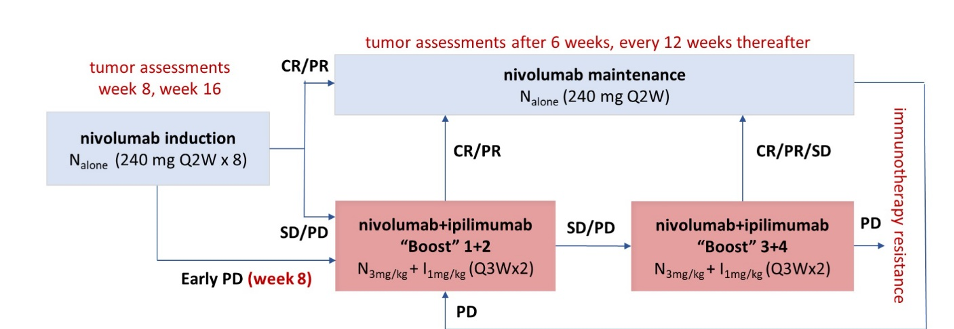
The authors assessed the primary endpoint of confirmed investigator-assessed objective response rate (ORR) per RECIST in both the first line (1L) and second-line (2L). Additional secondary endpoints included activity of nivolumab monotherapy, remission rate with nivolumab + ipilimumab “boost”, safety, and overall survival (OS).
They examined 109 patients without prior systemic therapy for kidney cancer and 98 patients in the second-line setting following prior treatment with tyrosine kinase inhibitors. The median age of included patients was 65 years (range 20-87) and 71 % had intermediate-risk disease and 25 % had poor-risk disease.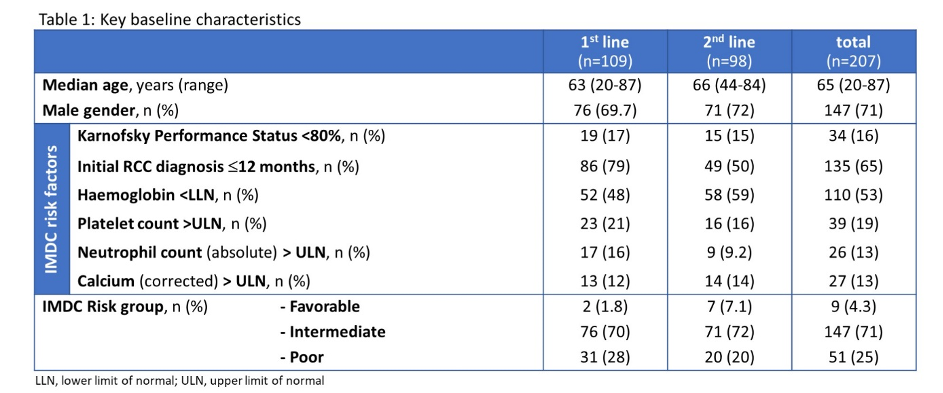
Confirmed ORR with nivolumab monotherapy was 28 % for patients receiving first-line therapy and 17 % for those being treated in the second-line. After a median follow-up of 12.8 months, the best overall response after nivolumab induction with or without nivolumab + ipilimumab was 36 % in previously untreated patients receiving first-line therapy and 30 % in the second-line therapy following first-line TKI. Among 38 patients who received nivolumab + ipilimumab for stable disease (SD) up to week 16, 1 (3 %) had a complete response, 4 (11 %) had a partial response, and 26 (68 %) had stable disease. In addition to those receiving nivolumab + ipilimumab for salvage disease, a further 28 patients in the first-line setting and 43 in the second-line setting received salvage nivolumab + ipilimumab for initial progressive disease. Of these patients with progressive disease, 3 (11 %) had a subsequent partial response and 8 (29 %) had stable disease in among those receiving first-line therapy, whereas 3 (7.0 %) achieved a complete response, 6 (14 %) had a partial response, and 13 (30 %) has stable disease in the second-line setting. A further 26 patients (16 in the first-line setting and 10 in the second-line setting) received salvage nivolumab + ipilimumab later than week 16 for progressive disease during nivolumab maintenance. Among the 16 patients with late progression in the first-line setting, 3 (19 %) achieved partial response and 5 (31 %) achieved stable disease, whereas, among the 10 with late progression in the second-line setting, 2 (20 %) achieved a partial response and 3 (30 %) achieved stable disease.
In the first-line setting, progression-free survival was 6.3 months (95 % CI 3.7 – 10.1) whereas t was 3.7 months (95 % CI 2.0 - 4.5) in the second-line setting. Overall survival was similarly longer in the first-line setting (27.2 months, 95 % CI 19.9 – not estimable (NE)) than the second-line setting (20.2 months, 95 % CI 15.6 – NE).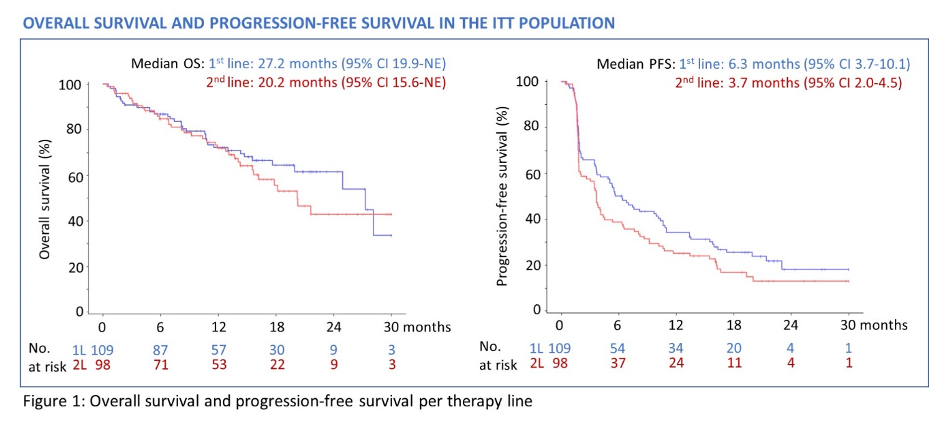
All-cause adverse events were seen in 99% of patients. Treatment-related adverse events occurred in 84% and 37% had grade 3 or 4 treatment-related adverse events. Three treatment-related adverse events occurred: cerebrovascular accident (first-line), respiratory failure (first-line), and pneumonia (second-line).
The authors conclude that this “tailored approach” with nivolumab + ipilimumab salvage for patients who fail nivolumab monotherapy improves response rates compared to nivolumab monotherapy alone.
Presented By: Marc-Oliver Grimm, MD, Professor, Otto-Schott-Institut der Friedrich-Schiller-Universität Jena, Jena, Germany
Written By: Christopher J.D. Wallis, MD, Ph.D., Urologic Oncology Fellow, Vanderbilt University Medical Center, Twitter: @WallisCJD at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


