NCT03401788 enrolled patients with known germline VHL alterations who had measurable and localized/nonmetastatic ccRCC, had not received prior systemic anticancer therapy and had ECOG PS 0 or 1. Once enrolled, patients received belzutifan 120 mg once daily until progression, intolerable toxicity, or decision to withdraw. The primary endpoint of this study is the objective response rate of VHL-associated ccRCC tumors per RECIST v1.1 by the independent review committee (IRC). Secondary endpoints include duration of response, time to response (TTR), PFS, and safety.
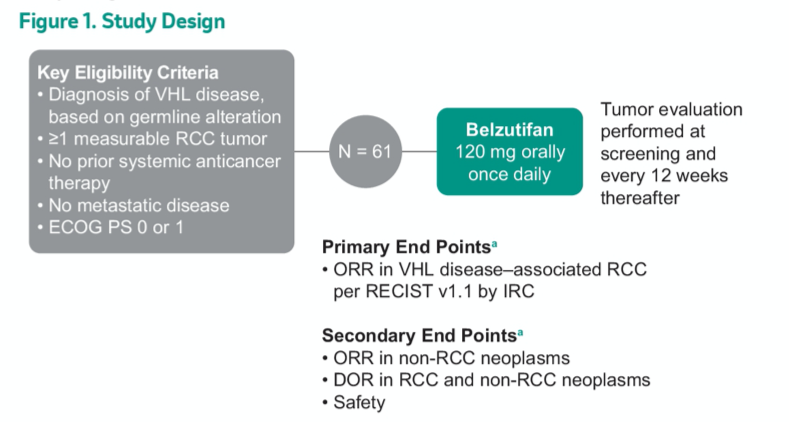
As of a data cut-off of December 1, 2020, this trial has enrolled 61 patients, the majority (82%) of whom have ECOG PS 0. The median number of prior tumor reduction procedures for any VHL-related lesion (eg, partial nephrectomy, craniotomy, radiation therapy) per patient was 5 (range, 0-15). In addition to renal lesions, pancreatic neuroendocrine tumors were present in 33% and CNS hemangioblastomas were present in 82% of included patients.
Over a median follow-up of 21.8 months (range 20.2-30.1), the objective response rate was 49.2% (95% CI 36.1-62.3%). There were 40 confirmed partial responses (ORR, 49.2%) and 4 (6.6%) unconfirmed responses. No patients had progressive disease.
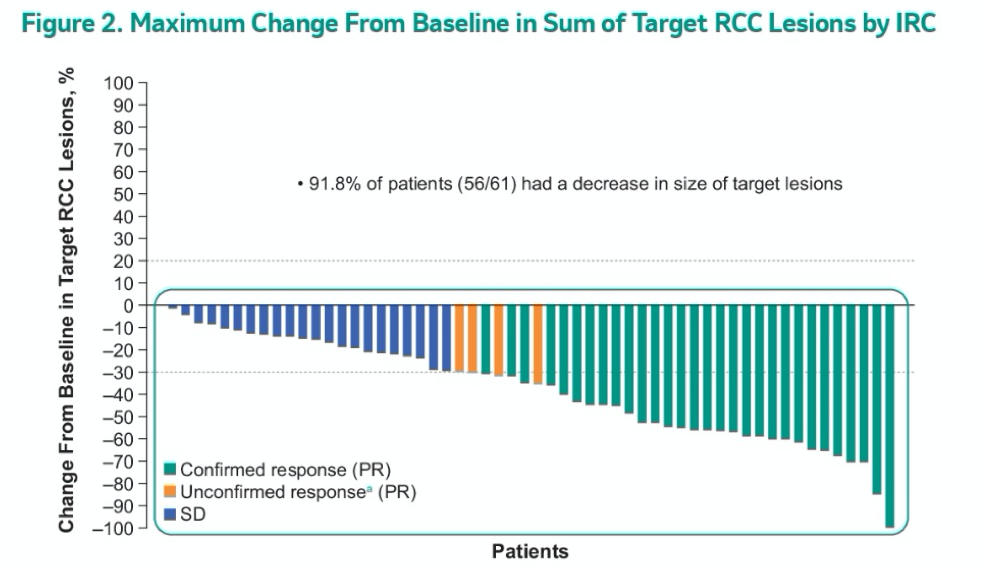
Among those with confirmed partial response, the median duration of response was not reached (2.8+ to 22.3+), the median time to response was 31 weeks (range, 12-61), and 56 pts (92%) had some reduction in the sum of all target lesion diameters.
At 52 weeks (1 year), the progression-free survival rate was 98% (95% CI, 89-100).
The authors further examined non-RCC tumors, for which the ORR was 77% (47/61; 6 CR) in pancreatic lesions, 91% (20/22; 3 CR) in pNETs, and 30% (15/50; 1 CR) in CNS hemangioblastomas. Additionally, 11 of the 16 pts with evaluable retinal hemangioblastomas at baseline showed improvement per IRC while the remaining had stable disease and none progressed.
All 61 enrolled patients (100%) had at least one adverse event, the most common of which (90%) was anemia. Treatment-related adverse events were reported by 60 of 61 patients (98%) and 8 (13%) had a grade 3 treatment-related adverse event though no patients had grade 4/5 events. A single patient discontinued treatment because of a treatment-related adverse event (grade 1 dizziness).
The authors concluded that belzutifan demonstrates clinical benefit and has a favorable safety profile in patients with VHL disease and associated tumors.
Presented by: Ramaprasad Srinivasan, MD, PhD, Investigator, Urologic Oncology Branch, Head, Molecular Cancer Therapeutics Section, National Cancer Institute, Center for Cancer Research
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


