To briefly summarize as JAVELIN Renal 101 has previously been presented and published, this trial accrued patients with advanced renal cell carcinoma and randomized them in a 1:1 fashion to avelumab and axitinib or sunitinib. In addition to clinical outcomes, blood samples were taken for biomarker analysis. In this analysis, the authors examined the median neutrophil-to-eosinophil ratio for patients both of the avelumab and axitinib and sunitinib arms as of the data cut-off of April 20, 2020, for the 3rd interim analysis. They examined progression-free survival (PFS), overall survival (OS), and objective response (OR), stratified by NER, and further performed multivariate Cox regression analyses of PFS and OS.
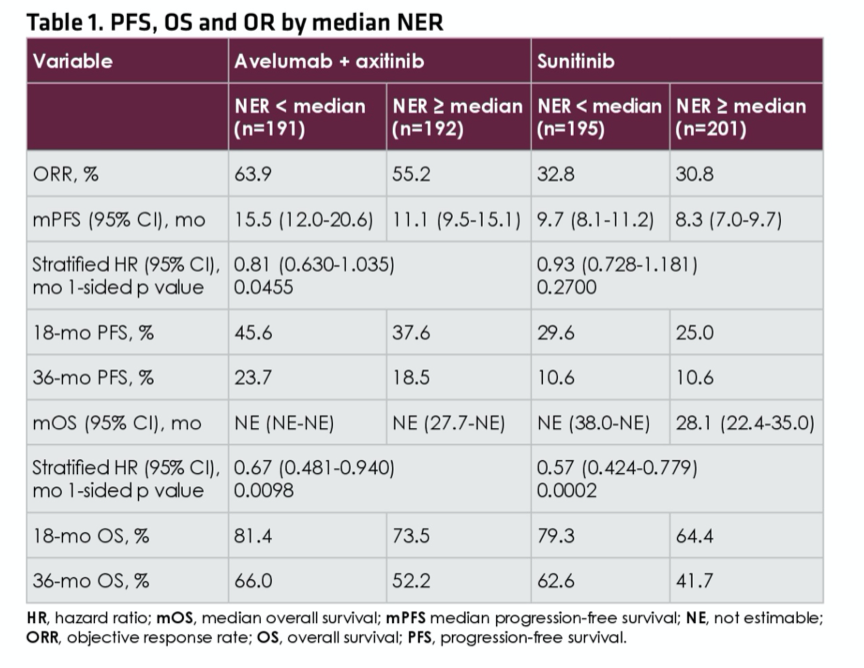
As of the data cut-off of April 20, 2020, the median neutrophil-to-eosinophil ratio for patients treated with avelumab and axitinib (n=383) was 29.2 and for patients treated with sunitinib (n=396) was 27.0. For patients randomized to avelumab and axitinib, objective response rate was higher (63.9% vs 55.2%) and median progression-free survival was longer (15.5 vs, 11.1 months) for patients with neutrophil-to-eosinophil ratio lower than the median, compared with above the median, for patients who were treated with avelumab and axitinib while there were no major differences in outcomes for patients treated with sunitinib.
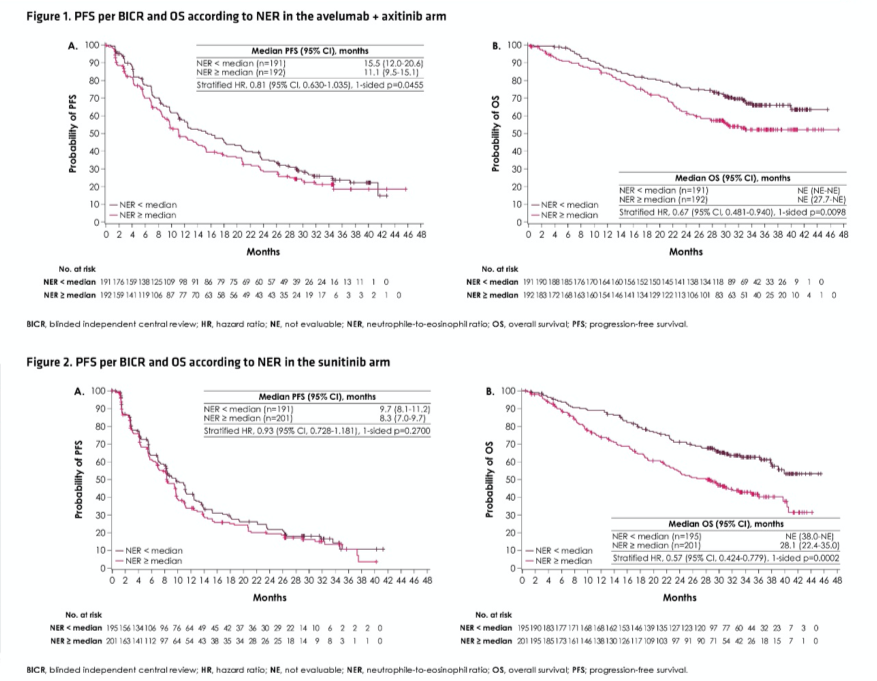
The authors then assessed the prognostic ability of neutrophil-to-eosinophil ratio in multivariable-adjusted models. Assessing progression free survival, the stratified hazard ratio for patients with neutrophil-to-eosinophil ratio below the median, compared to equal to or above the median, was 0.81 (95% CI, 0.630-1.035) for patients treated with avelumab and axitinib and 0.93 (95% CI, 0.728-1.181) for those treated with sunitinib. Similarly, assessing overall survival, the stratified hazard ratio for patients with neutrophil-to-eosinophil ratio below the median, compared to equal to or above the median, was 0.67 (95% CI 0.481-0.940) for patients treated with avelumab and axitinib and 0.57 (95% CI, 0.424-0.779) for those treated with sunitinib.
The significant association between neutrophil-to-eosinophil ratio and these survival outcomes was also demonstrated when operationalized continuously, in addition to this dichotomous fashion.
The authors concluded that neutrophil-to-eosinophil ratio may be prognostic for overall survival in patients with advanced renal cell carcinoma, and potentially predictive of improved response among those treated with avelumab and axitinib.
Presented by: Matthew D. Tucker, MD, Division of Hematology/Oncology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


