For the IMMUNOPRESERVE trial, patients with localized muscle-invasive bladder cancer in clinical stages T2-4a N0 M0, ECOG 0-1, without contraindications to immunotherapy, who either wished for bladder preservation or were ineligible for cystectomy, were included in this phase II study. Treatment consisted of initial transurethral resection (TUR) of the tumor, followed by durvalumab 1,500 mg IV plus tremelimumab 75 mg IV, every 4 weeks for 3 doses. Normofractionated external-beam radiotherapy was started 2 weeks later, at doses of 46 Gy to the minor pelvis and 64-66 Gy to the bladder. Patients with either residual or relapsed muscle-invasive bladder cancer were offered salvage cystectomy. The trial schema for IMMUNOPRESERVE is as follows:

The primary endpoint was complete response defined as the absence of muscle-invasive bladder cancer at post-treatment tumor site biopsy. Secondary endpoints included disease-free survival, overall survival, bladder intact disease-free survival, safety, and long-term function, and late sequelae in preserved bladders. A 2-stage sequential design was used (complete response rate P0=5, P1=0.7, α=0.10, β=0.20) requiring at least six complete responses in the first 12 patients to expand to a second cohort of 20 patients.
From January 2019 to August 2020, 32 patients were enrolled at six centers. The median age was 71 years (IQR 49-91). Performance status was 0 in 24 patients and 25 patients were male. The clinical-stage was T2 in 28 patients, T3 in 3, and T4a in 1 patient. All patients received at least two immunotherapy cycles. The median dose of radiotherapy administered was 64 Gy (IQR 50-65). Complete response at post-treatment biopsy was documented in 26 (81%) patients, two patients had residual muscle-invasive bladder cancer and four patients were not evaluated due to rejection (n = 1), clinical impairment (n = 1), death from COVID 19 (n = 1) and a suspected treatment-related death from peritonitis (n = 1). Over a median follow-up of 12.7 months (IQR 5.3 – 24.5 months), two patients underwent salvage cystectomy because of muscle-invasive bladder cancer and T1 relapses, respectively. The estimated 12-month disease-free survival (DFS) rate was 76% (95% CI 62%-94%):
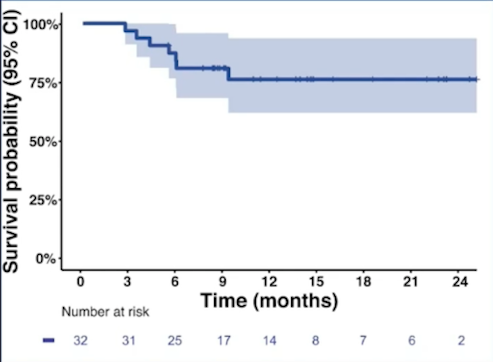
The estimated 12-month overall survival (OS) rate was 76% (95% CI 62%-94%):
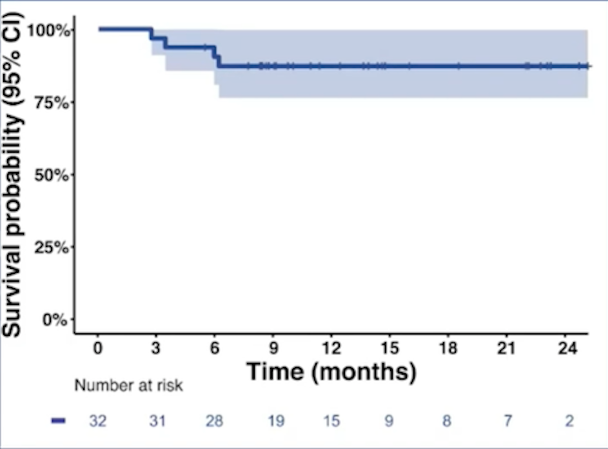
The estimated 12-month bladder intact disease-free survival (DFS) rate was 73% (95% CI 59%-91%):

The 12-month non-muscle-invasive bladder cancer (NMIBC) local failure rate was 6% (95% CI 1-19%), the 12-month muscle-invasive bladder cancer local failure rate was 14% (95% CI 4-30%), and the 12-month distant metastasis rate was 12% (95% CI 3-29%).
A total of 31 (97%) patients experienced adverse events related to radiotherapy and/or immunotherapy, with diarrhea (41%) and urinary disorders (37%) as the most frequent. Grade 3 or 4 adverse events related to therapy were reported in 31% of patients, with the most frequent being diarrhea (12%), acute kidney failure (6%), and hepatitis (6%).
Dr. Garcia del Muro concluded his presentation of IMMUNOPRESERVE with the following take-home messages:
• The combined-modality bladder-preserving approach including durvalumab + tremelimumab with concurrent radiotherapy is feasible and safe
• This therapeutic regimen showed high efficacy in terms of response and elicited bladder preservation in a large number of patients
• Longer follow-up is still needed, focusing on long-term bladder preservation and survival
• Further research on this approach as an alternative to cystectomy in selected patients with localized muscle-invasive bladder cancer is warranted
Clinical trial information: NCT03702179
Presented By: Xavier Garcia del Muro, Department of Medical Oncology. Institut Català d'Oncologia (ICO) L'Hospitalet del Llobregat, Barcelona, Spain
Co-Authors: Begoña P. Valderrama, Ana Medina, M. Andres Cuellar, Olatz Etxaniz, Regina Gironés Sarrió, María Jose Juan-Fita, Ferran Ferrer, Isabel Miras Rodríguez, Guillermo Lendínez-Cano, Roberto de Haro Piedra, Arturo Candal Gomez, Venancio Chantada Abal, Oscar Buisán, Salvador Villà, José Luis Pontones, Erica Collado, José L. Domínguez-Escrig, Yashmina Murria, Francesc Vigués, SOGUG-Spanish Oncology Genitourinary Group; Medical Oncology. Institut Català d'Oncologia (ICO) L'Hospitalet del Llobregat, Barcelona, Spain; Department of Medical Oncology, Hospital Universitario Virgen del Rocío, Seville, Spain; Medical Oncology. Fundación Centro Oncológico de Galicia, A Coruña, Spain; Medical Oncology. Institut Català d'Oncologia (ICO) Hospital Germans Trias i Pujol, Badalona, Spain; Medical Oncology Service, Hospital Universitari i Politècnic la FE, Valencia, Spain; Medical Oncology Service. Instituto Valenciano de Oncología, Valencia, Spain; Radiotherapy Oncology, Institut Català d'Oncologia (ICO) L'Hospitalet del Llobregat, Barcelona, Spain; Medical Oncology. Hospital Universitario Virgen del Rocío, Seville, Spain; Urology Service, Uro-Oncology Unit. Hospital Universitario Virgen del Rocío, Sevilla, Spain; Radiotherapy Oncology, Hospital Universitario Virgen del Rocío, Seville, Spain; Radiotherapy Oncology, Fundación Centro Oncológico de Galicia, A Coruña, Spain; Urology Department. Fundación Centro Oncológico de Galicia, A Coruña, Spain; Urology Department. Institut Català d'Oncologia (ICO) Hospital Germans Trias i Pujol, Badalona, Spain; Radiation Oncology Department, Catalan Institute of Oncology, Badalona, Barcelona, Spain; Oncology Urology Department. Hospital Universitari i Politècnic la Fe, Valencia, Spain; Medical Oncology. Hospital Universitari i Politècnic la FE, Valencia, Spain; Urology Service, Fundación Instituto Valenciano De Oncología (I.V.O.), Valencia, Spain; Radiation Oncology Department, Fundación Instituto Valenciano de Oncología (I.V.O.), Valencia, Spain; Urologist. Hospital Universitari de Bellvitge, Barcelona, Spain
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


