To briefly summarize the previously reported and published TITAN study design (NCT02489318), 1052 patients with mCSPC were randomized 1:1 to apalutamide (240 mg daily; n = 525) or placebo (n = 527) along with ongoing ADT. 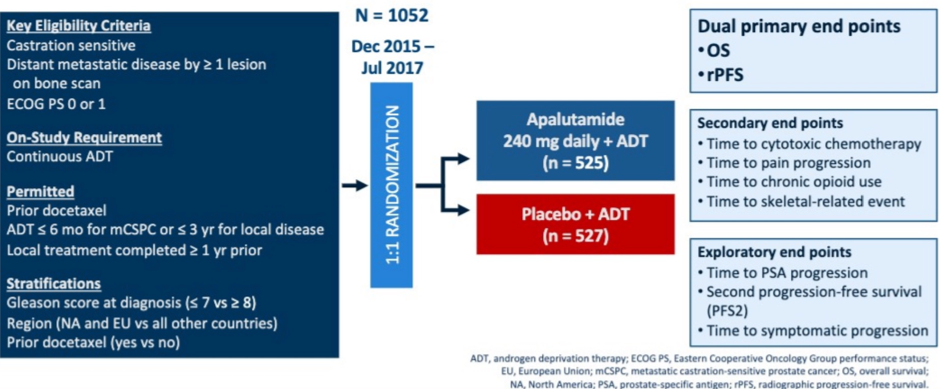
As a key secondary outcome, patient-reported outcomes were assessed using Brief Pain Inventory-Short Form (BPI-SF) and Functional Assessment of Cancer Therapy-Prostate (FACT-P). BPI was completed for 7 days consecutively (Days -6 to 1 of each 28-day cycle) through end of treatment. FACT-P was completed at baseline (BL), cycles 2 to 7, and then every other cycle through the end of treatment. Mean scores were reported by treatment group and over time. Time to deterioration on BPI and FACT-P scores was calculated by Kaplan-Meier methods and compared between groups using proportional hazards regression models.
Assessed each cycle, more than 62% of eligible patients completed BPI through cycle 32 and more than 50% completed FACT-P through cycle 31. In both groups, patients were relatively asymptomatic at the time of enrollment and randomization with good baseline HRQoL: on the worst pain severity scale (BPI) measured from 0-10, median scores were 1.1 (apalutamide) and 1.0 (placebo); on a HRQoL scale measured from 0-156 (FACT-P total; higher score = better HRQoL), median scores were 113.0 (apalutamide) and 113.3 (placebo).
Low baseline BPI scores remained stable over time in both groups. 
On average, favorable baseline FACT-P scores did not notably worsen over time either among patients who received apalutamide or those who received placebo. 
Further, the median time to deterioration in any BPI or FACT-P scores did not significantly differ between treatment groups.
Assessed each cycle, at least 86% of patient treated with apalutamide and 85% of those treated with placebo were either “not at all” or “a little bit” bothered by side effects. At baseline, 76% of patients receiving apalutamide and 72% of those receiving placebo had favorable energy levels (reporting lack of energy “not at all” or “a little bit”). Similarly, energy levels remained stable or improved at each cycle for the majority of patients, regardless of treatment allocation.
The authors conclude that this final analysis of the TITAN trial demonstrates that, in addition to improved overall and radiographic progression free survival with the addition of apalutamide to ADT in mCSPC, there is not appreciable difference in patient-reported HRQoL or side effect burden.
Presented by: Neeraj Agarwal, MD, Professor in the Division of Oncology, Department of Medicine, at the Huntsman Cancer Institute (HCI) at the University of Utah School of Medicine. He is the Huntsman Cancer Institute (HCI) Presidential Endowed Chair of Cancer Research, and the Director of the Genitourinary Oncology Program, Dr. Agarwal also serves as the physician-scientist and senior director of clinical research innovation at HCI, Salt Lake City, Utah
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
Related Content: Apalutamide Confirms Overall Survival Benefit at 44-Months in Metastatic Castration-Sensitive Prostate Cancer - The TITAN Study - Neeraj Agarwal


