The authors performed a multicenter international randomized phase 3 trial in which 350 men with T1c-4N0M0 unfavorable-risk prostate cancer were assigned in a 1:1 ratio to receive ADT plus radiotherapy and docetaxel (60 mg/m2 q3 weeks for 3 cycles before radiotherapy and 20 mg/m2 weekly during radiotherapy) versus ADT+ radiotherapy without docetaxel. The authors collected data regarding second cancer incidence and survival status. The primary endpoint was overall survival though the authors also assessed the incidence of RT-induced cancers. Analytically, they explored whether the treatment effect of docetaxel on overall survival differed within PSA subgroups (< 4, 4-20, and >20 ng/mL) using the interaction test for heterogeneity adjusted for age and known prostate cancer prognostic factors.
With a median follow-up of 10.2 years, 89 men died (25.43%), of whom 42 died of prostate cancer (47.19%). The authors found no significant difference in overall survival between those who received docetaxel (restricted mean survival time over 10-years = 9.11 years) compared to those with received ADT and radiotherapy (8.82 years) with a difference of 0.29 (95% CI: -0.19, 0.76) years (p = 0.22). However, there were significantly fewer radiotherapy-induced cancers among those who received docetaxel [10-year estimates: 0.61% versus 4.90%: age-adjusted HR of 0.13: 95% CI: 0.02, 0.97; p = 0.046].
Assessing for effect modification between PSA groups, men with a PSA < 4 ng/mL versus 4-20 ng/mL had a significantly larger benefit from the addition of docetaxel to ADT and radiotherapy on overall survival [adjusted HR: 0.27, 1.51; p-value for interaction = 0.04].
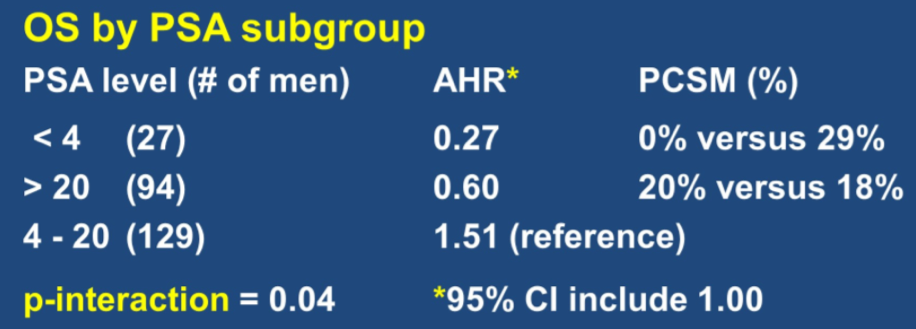
This was driven by a lower rate of prostate cancer-specific mortality among men randomized to docetaxel [0/13 (0.00%) versus 4/14 (28.57%)] among men with PSA < 4 ng/mL.

Thus, the use of docetaxel, with ADT and radiotherapy, reduced rates of radiotherapy-induced cancers. This is a novel observation.
The authors concluded that the addition of docetaxel to ADT and radiotherapy in men with unfavourable risk prostate cancer did not improve overall survival, though there may be a beneficial effect among men with PSA <4 ng/mL due to decreased prostate cancer related mortality, though this is a post-randomization, hypothesis-generating observation.
Presented by: Anthony D'Amico, MD, PhD, Professor, Radiation Oncology, Harvard Medical School, Chief, Genitourinary Radiation Oncology, Brigham And Women's Hospital, Chief, Genitourinary Radiation Oncology, Dana-Farber Cancer Institute, Boston, MA
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


