The authors enrolled men with asymptomatic or mildly symptomatic metastatic CRPC to ODENZA, a prospective, randomized, open-label, multicenter, cross-over, phase II trial of preference between darolutamide and enzalutamide (NCT03314324). Randomization was performed in a 1:1 fashion to darolutamide (1200 mg daily) for 12 weeks followed by enzalutamide 160 mg daily for 12 weeks or the reverse sequence with enzalutamide followed by darolutamide. In both arms, the second treatment was given in absence of evidence of cancer progression at week 12.

The primary endpoint was patient preference between the two drugs, as assessed by a questionnaire at week 24. The Prescott's test was used to determine treatment preference among patients who had exposure to both treatments, no progression at week 12, and completed the preference questionnaire. Stratification factors were performance status and prior taxane for mCSPC. After week 24, patients went on to an extension period during which they received their chosen treatment until progression or toxicity. The main secondary objectives included reasons for preference, response at week 12, cognitive assessment, and toxicity.
The authors enrolled and randomized 249 patients, who had a median age of 72 years (68; 79) and the majority (56%) had a baseline ECOG PS of 0. Just under one-quarter (22%) of patients had prior taxane therapy.
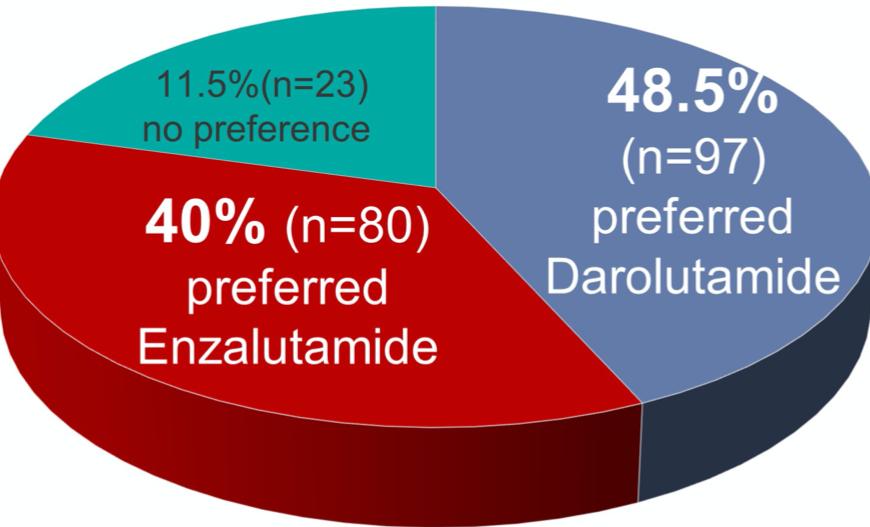
Assessing the primary outcome, 200 patients fulfilled the pre-planned criteria for evaluation of the preference primary endpoint: 97 (48.5% [95% confidence interval 41.3 - 55.7]) preferred darolutamide, 80 (40.0% [95% confidence interval 33.0 - 47.0]) preferred enzalutamide, and 23 (11.5% [95% confidence interval 6.8 - 16.2]) had no preference (unilateral p-value of 0.92).
After preference assessment, 186 patients continued on to the extension period with 103 (55.4%) patients choosing darolutamide and 83 (44.6%) choosing enzalutamide.
All expressed reasons for patient preference numerically favoured darolutamide over enzalutamide, though none were statistically significant. These reasons included less fatigue (44% vs 29%), ease of taking the medication (37% vs 31%), better quality of life (36% vs 28%), ability to be more active (26% vs 15%), ability to concentrate (22% vs 15%) and less falls (6% vs 3%).

Fatigue was the most frequently reported all-grade adverse event at week 12, reported in 21% of patients taking darolutamide and 36% taking enzalutamide.
Assessing oncologic outcomes, a PSA50 response was achieved in 76.2% and 83.9% at week 12 with darolutamide and enzalutamide respectively, a non-significant difference (p = 0.13).
The authors concluded that a numerically greater number of patients with early mCRPC preferred darolutamide compared with enzalutamide, though this was not significantly different. Fatigue was a key factor influencing patient preference.
Presented by: Emeline Colomba, Institut de Cancérologie Gustave Roussy, Villejuif, France
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


