(UroToday.com) The 2021 ASCO annual meeting’s poster discussion session for prostate cancer included a discussant presentation by Dr. Julie Graff discussing novel approaches to metastatic prostate cancer.
Dr. Graff notes that over the last decade we have made significant strides in the treatment of metastatic castration-resistant prostate cancer (mCRPC) with several new agents available, relying heavily on the androgen receptor inhibitors, namely enzalutamide and abiraterone:
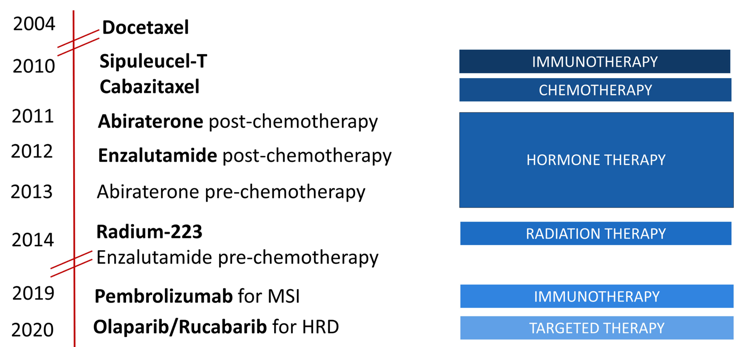
However, it is important to note, that there are still no cures in mCRPC, just incremental increases in overall survival.
The first abstract discussed by Dr. Graff was by Dr. Scott Tagawa “Phase I study of 225Ac-J591 for men with mCRPC”. Dr. Graff notes that radiopharmaceutical successes date back to 1949 with the advent of radioactive iodine therapy for patients with metastatic thyroid cancer. However, the successes in prostate cancer have been limited, with only one approved therapy, Radium-223. In the current study, heavily pre-treated mCRCP patients received a single infusion of 225Ac-J591 (13.3 KBq/kg with planned escalation up to 93.3 KBq/kg). Dose-limiting toxicity was defined as attributable grade 4 hematological toxicity or grade 3/4 non-hematological toxicity. There were 32 men treated with a single dose of 225Ac-J591 on 7 dose levels with expansion at the highest dose level (n = 16). The median age was 69.5 (range 52-89) years and median PSA was 149.1 ng/mL (range 4.8-7168.4). There were 75% of patients with >2 prior AR-pathway inhibitors, 62.5% had previous chemotherapy, 28% previous Radium-223, and 43.7% previous 177Lu-PSMA. While PSMA uptake was not a prerequisite for treatment, among 28 patients with pre-treatment PSMA PET:
- None had a tumor SUVmax < liver
- 5 (17.8%) with tumor SUVmax 1-2.5x liver
- 2 (7.2%) with tumor SUVmax5-5x liver
- 21 (75%) with tumor SUVmax > 5x liver SUVmean
One of six patients in cohort 6 (80 KBq/kg) had dose-limiting toxicity (grade 4 anemia and platelets) with 0 of 6 patients at the highest dose level (93.3 KBq/Kg) and thus this dose was expanded. High grade adverse events were restricted to hematologic: in addition to dose-limiting toxicity, 4 (12.5%) patients with grade 3 platelets and 2 (6.2%) patients with grade 3 neutropenia. Non-hematological adverse events were restricted to grade 1/2 and included: 10 patients (31.2%) with fatigue, 5 patients (15.6%) with pain flare, 14 patients (43.7%) with nausea, 8 patients (25%) with grade 1 xerostomia (of which 5 received prior 177Lu-PSMA), and 12 patients (37.5%) with AST elevation. Despite prior treatment including 177Lu-PSMA and no selection for PSMA expression, 22 (68.8%) patients had any PSA decline, and 14 (43.8%) had > 50% PSA decline:
Among 21 patients with paired baseline and 12-week circulating tumor cell counts, 12 patients declined (5 converting from unfavorable to favorable and 5 converting detectable to 0), 5 patients remained 0, and 4 patients increased their circulating tumor cell count. In the subset with PRO data, pain scores by BPI-SF tended to improve by week 12. Following a single dose of 225Ac-J591, median PFS was 7.2 months (95% CI 4.6-not reached), and median OS was 10.9 months (95% CI 7.6-21.1). Dr. Graff notes that being able to track PSA and circulating tumor cells is important for assessing efficacy of treatment.
The second study discussed was by Dr. Mark Markowski “COMBAT-CRPC: COncurrent adMinistration of Bipolar Androgen Therapy and nivolumab in men with mCRPC”. This study treated men with mCRPC with testosterone cypionate 400mg intramuscular (BAT) every 28 days and nivolumab 480mg IV every 28 days, during which time LHRH agonist treatment was continued. All patients received BAT as single agent therapy for a 12-week lead-in prior to the addition of nivolumab. The trial design for this study is as follows:
There were 45 patients enrolled on study and treated. The confirmed PSA50 response rate was 40.0% (n = 18/45, 95% CI: 26-56%, p = 0.02 against the 25% null hypothesis). For patients with measureable disease, the objective response rate was 23.8% (n = 10/42):
Median rPFS on BAT and nivolumab was estimated at 5.7 months (95% CI 4.9-7.8 months), and 11.1% (n = 5/45) of patients were free from radiographic progression for 11 or more months. One patient achieved a complete radiographic response, which is ongoing (>13 months):
The majority of adverse events were Grade <2. The most common adverse events were edema (20%), nausea (20%), and back pain (13%). Immune-related adverse events were generally mild (Grade <2) with only two grade 3 immune related adverse events observed (pericarditis, lipase elevation).
The final study discussed by Dr. Graff was presented by Dr. Johann de Bono entitled “Results of an ongoing phase 1/2a dose escalation study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager, in patients with mCRPC.” This Ph1/2a study is evaluating HPN424 in mCRPC patients who have received > 2 prior systemic therapies. The primary endpoints are safety, tolerability and determination of maximum tolerated dose and the recommended phase 2 dose. Secondary objectives include: pharmacokinetics, pharmacodynamics, immunogenicity and preliminary anti-tumor activity. HPN424 is administered IV once weekly, and tumor assessments include PSA, CT and bone scans every 9-weeks. As of April 23, 2021, 89 patients were dosed in 15 cohorts with target doses ranging from 1.3 to 160 ng/kg fixed dose, and up to 300 ng/kg with step dosing to the target dose after initial priming dose. Patients had received a median of 5 prior systemic regimens (a median 2 prior novel hormonal agents), with 73% having received prior chemotherapy for mCRPC. The median age was 70 (43 – 91) years. The most common grade > 3 treatment related adverse events were AST increase (21%), anemia (11%) and ALT increase (16%). Dose limiting toxicities included grade 3 cytokine release syndrome (n = 4), grade 3 elevated lipase (n = 1) and grade 3 seizure (n = 1). These events did not limit escalation, as the maximum tolerated dose has not been reached and escalation continues. All grade cytokine release syndrome occurred in 69% of patients, with grade 3 events occurring after first administration of target dose (n = 2 fixed dose, n = 1 step dose). Transaminase elevation occurred predominantly during Cycle 1, was transient and had no clinical sequelae. Disease progression was the primary reason for drug discontinuation, with two patients (3%) discontinuing treatment secondary to treatment related adverse events.
Reduction in circulating tumor cells was seen in 36 of 64 patients (56%) with measurable circulating tumor cells at baseline, including 14 patients who had a CTC0 response. Fifteen of 74 patients with at least 6 months of follow-up have remained on treatment beyond 24 weeks, and one patient experienced confirmed partial response at 160 ng/kg. Fifteen of 74 patients (20%) with >=1 post-baseline value had PSA decreases from baseline ranging from -2% to -76%, including four patients with PSA50 response and two patients with PSA30 response:
In chemotherapy-naïve patients, 6 of 20 (30%) showed PSA declines post-baseline, including three with PSA50 and one with PSA30 response. Eight of 17 patients (47%) chemotherapy-naïve patients in the castrate-resistant state with at least six months of follow-up have remained on treatment beyond 24 weeks: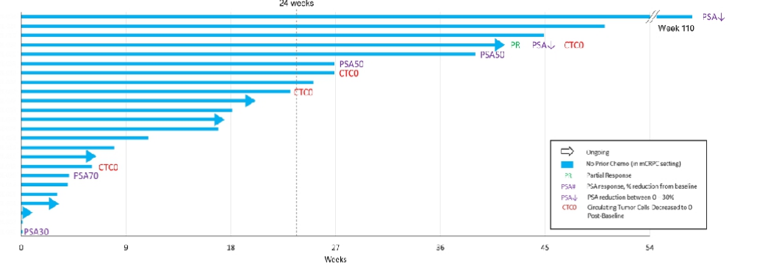
There were 41 patients of 89 (46%) that had measurable disease at baseline, including 34 patients with >=1 post-treatment protocol scheduled disease assessment. Among these 34 patients, the sum of target lesions in 19 patients (56%) remained stable or showed reduction, including 1 confirmed partial response. Dr. Graff notes that it is important that these trialists have been able to manage the cytokine release syndrome and looks forward to seeing the results of the expansion study.
In conclusion, Dr. Graff states that yes, in fact she does believe that we are making progress with novel approaches in metastatic prostate cancer, and encourages clinicians to enroll patients in these important early phase clinical trials.
Presented by: Julie N. Graff, MD, OHSU Knight Cancer Institute, Portland, OR
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


