This study implemented a scalable strategy using low coverage whole genome sequencing of formalin fixed paraffin embedded diagnostic core biopsies from STAMPEDE participants randomized to the standard of care ADT arm, between 2005 and 2016. There were 315 cases randomly selected, aiming for a biomarker population of 300, anticipating an assay failure rate ̃of 5%. 40% was defined as the minimum histopathologically determined tumor cellularity for inclusion. Survival analysis was performed investigating percentage genome altered at diagnosis as a continuous measure with fractional polynomial specification in Cox models adjusting for disease burden, Gleason grade, pre-ADT PSA (log-transformed), age at randomization, and tumor cellularity. It was pre-specified that all hypothesis tests required evidence at the 5% significance level to consider rejecting the null hypothesis.
Successful copy number profiling was achieved in 300/315 cases. There was no significantly different baseline clinico-pathological features between the full trial comparison n = 3,106 and final biomarker population n = 300; 290/300 cases were de novo presentations. Percentage genome altered in the core with highest Gleason grade and tumor cellularity was median 18% (range 0%-75%; n = 300). Percentage genome altered was significantly higher in M1 (n = 169) compared to M0 (n = 131) cases (median: 21% vs 14%; p = 0.00006). There were 284/300 patients that were sub-classified by disease burden into M0 node-negative and node-positive and M1 low and high volume. Percentage genome altered was significantly associated with increased disease burden (p = 0.00002). Increased percentage genome altered was significantly and non-linearly associated with an increased hazard of failure-free survival (p = 0.004), progression-free survival (p = 0.002), metastatic progression-free survival (p = 0.003), overall survival (p = 0.045) and prostate cancer-specific survival (p = 0.011):
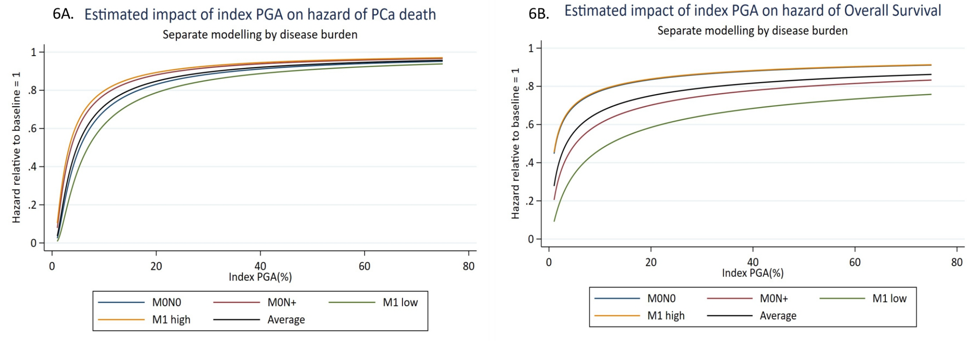
Dr. Grist concluded her presentation of the STAMPEDE trial molecular risk stratification with the following summary points:
- Evaluation of the burden of copy aberrations in archival, poor quality formalin fixed paraffin embedded diagnostic tissue from men randomized in the STAMPEDE trial is feasible using low coverage whole genome sequencing
- Increased copy number alteration in advanced prostate cancer is significantly and non-linearly associated with increased hazard of death
Presented by: Emily Grist, University College London Cancer Institute, London, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


