Patients with mCRPC with ongoing ADT and no prior life-prolonging treatment were randomized 1:1 to apalutamide (240 mg QD) + abiraterone acetate (1000 mg QD) + prednisone (5 mg BID) or placebo + abiraterone acetate + prednisone. Randomization was stratified by presence or absence of visceral disease, ECOG performance status 0 or 1, and geographic region. The trial schema for ACIS is as follows:
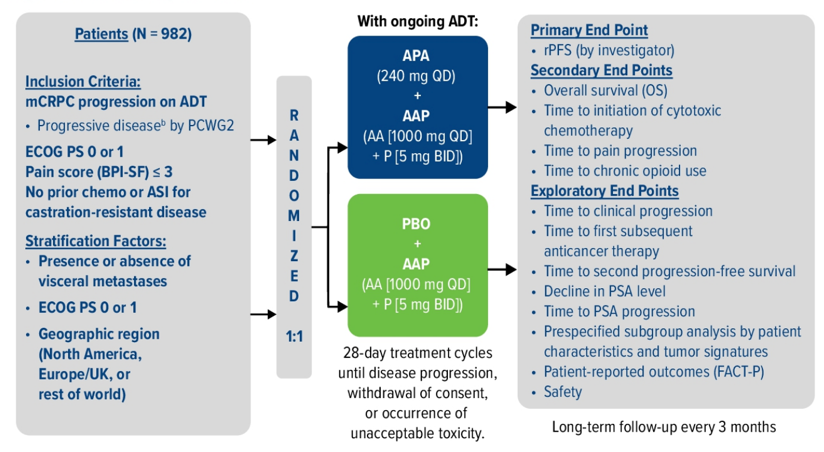
The primary endpoint for this trial was rPFS (randomization to radiographic progression or death), and secondary endpoints included overall survival (OS), time to initiation of cytotoxic chemotherapy, time to chronic opioid use, time to pain progression, and safety.
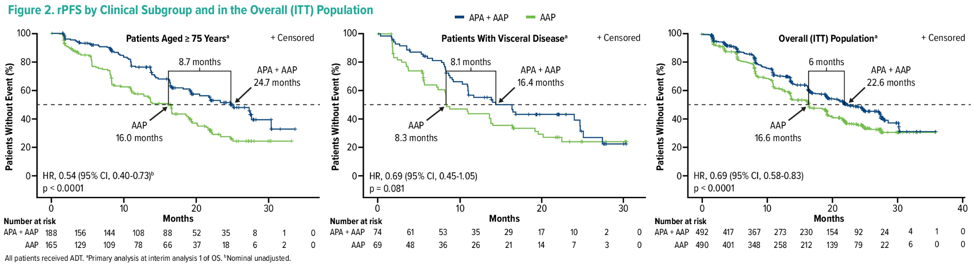
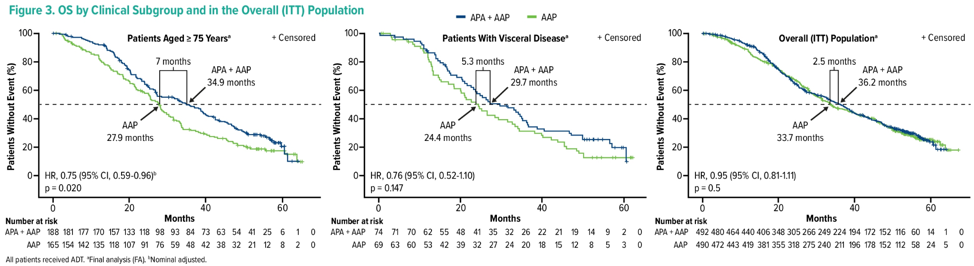
There were 982 patients enrolled of which 14.6% had visceral disease and 35.9% were ≥ 75 years of age. Median rPFS, OS, and time to pain progression favored apalutamide + abiraterone acetate + prednisone versus placebo + abiraterone acetate + prednisone (HR < 1) in both subgroups. For patients ≥ 75 years of age, rPFS, and OS were ≥ 7 months longer with apalutamide + abiraterone acetate + prednisone.
rPFS:
OS:
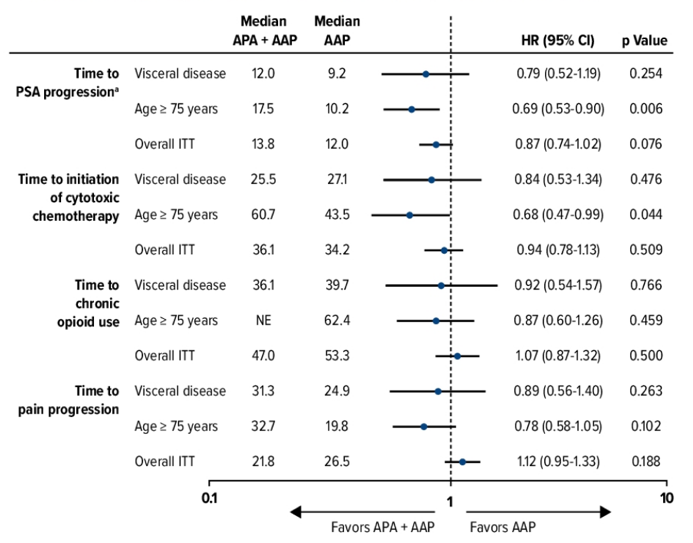
Secondary and other endpoints by clinical subgroup:
Overall, treatment-emergent adverse events were similar (all > 94%) in patients with visceral disease, those ≥ 75 years of age, and overall safety population. Hypertension was more frequent with apalutamide + abiraterone acetate + prednisone versus placebo + abiraterone acetate + prednisone mainly in patients ≥ 75 years of age (31.7% vs 17.6%). Grade 3/4 treatment-emergent adverse events (apalutamide + abiraterone acetate + prednisone versus placebo + abiraterone acetate + prednisone) were as follows:
- Visceral disease, 60.8%, n = 74 vs 48.5%, n = 68
- ≥ 75 years of age, 71.5%, n = 186 vs 68.5%, n = 165
- Overall, 63.3%, n = 490 vs 56.2%, n = 489
Treatment-emergent adverse events leading to discontinuation (apalutamide + abiraterone acetate + prednisone versus placebo + abiraterone acetate + prednisone) were as follows:
- Visceral disease, 17.6% vs 5.9%
- ≥ 75 years of age, 26.3% vs 20.6%
- Overall, 16.9% vs 12.5%
Finally, treatment-emergent adverse events leading to death (apalutamide + abiraterone acetate + prednisone versus placebo + abiraterone acetate + prednisone) were as follows:
- Visceral disease, 6.8% vs 5.9%
- ≥ 75 years of age, 5.4% vs 13.9%
- Overall, 3.5% vs 7.6%.
Dr. Saad concluded his presentation of the ACIS subgroup analysis with the following take-home messages:
- In this analysis of two difficult to treat mCRPC subgroups (≥ 75 years of age and visceral disease) in the ACIS study, addition of apalutamide + abiraterone acetate + prednisone favored rPFS and OS compared to placebo + abiraterone acetate + prednisone
- Safety, while generally consistent with the overall population, showed higher hypertension rate in patients ≥ 75 years of age and treatment-emergent adverse events leading to discontinuation in patients with visceral disease
Clinical trial information: NCT02257736
Presented by: Fred Saad, MD, FRCS, Centre Hospitalier de l'Université de Montréal, Montréal, QC, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
Related Content:
ASCO GU 2021: Final Results from ACIS, a Randomized, Placebo-Controlled Double-Blind Phase 3 Study of Apalutamide and Abiraterone Acetate plus Prednisone (AAP) Versus AAP in Patients with Chemo-Naive Metastatic Castration-Resistant Prostate Cancer


