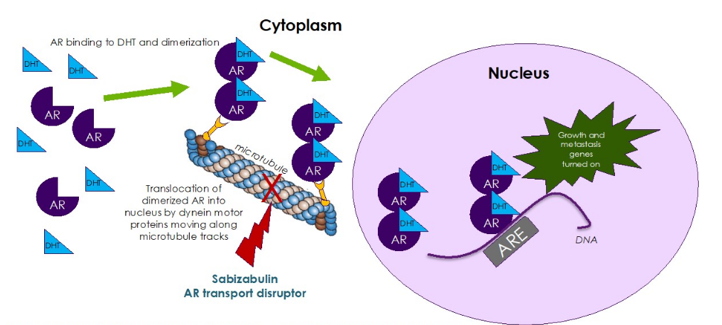
(UroToday.com) Sabizabulin (VERU-111) is an oral cytoskeletal disruptor that disrupts microtubules supporting the cytoskeleton and has no affinity for multidrug resistance proteins. Additionally, it has been shown to cleave poly (ADTP-ribose) polymerase (PARP) protein, with a favorable toxicity profile with no neurotoxicity and no neutropenia or myelosuppression. The following schematic depicts sabizabulin targeting microtubules and disrupting transport of androgen receptors into the nucleus:
At the 2021 ASCO annual meeting, Dr. Mark Markowski presented results of the phase 1b/2 clinical study establishing the maximum tolerated dose and evaluating the preliminary efficacy in men with mCRPC also resistant to androgen receptor targeting agents.
In the phase 1b component of the study, a 3+3 design with escalating oral dosing of 4.5 mg to 81 mg (7 days on drug/14 days off per 21-day cycle) was utilized. The schedule was also expanded to continuous dosing/cycle. The phase 2 portion utilized 63 mg daily dosing to evaluate efficacy in ~40 taxane-naïve men with mCRPC that have failed at least one androgen receptor targeting agent.
In the phase 1b portion of the study, 30 taxane-naïve men with mCRPC and a median age of 76 (61-92) were enrolled, of which 8 had received prior enzalutamide, 12 prior abiraterone and 10 both prior therapies. There were 8 men that had bone metastasis, 5 patients with lymph node metastasis, 5 with mixed locations of metastasis, and 1 had soft tissue metastases at study entry. The maximum tolerated dose of VERU-111 was 72 mg (3/11 men had grade 3 diarrhea) and the recommended phase 2 dose was 63 mg. Grade 3 diarrhea was not observed at doses ≤ 63 mg per day and the most common non-dose limiting adverse events were mild to moderate nausea, vomiting, diarrhea, and fatigue, with no observed neurotoxicity or neutropenia. Efficacy was assessed by PSA and bone/CT scans. In men treated for ≥ 4 continuous 21-day cycles, 6/10 (60%) had PSA declines, 4(40%) men had ≥ 30% declines and 2(20%) ≥ 50% declines compared to their 21-day cycle baseline PSA. Median PFS is currently 12 months (6-23+ months) with 3 patients continuing on study, two of which have been on study for approximately 2 years. In patients receiving at least a single dose of ≥ 63 mg daily (n=19), objective tumor responses were seen in 3 men (16%). The median rPFS in these patients is currently 12.4 months.
In the phase 2 portion of the study (n = 41), 55% of the patients had bone only metastases, 11% had nodal only, 32% had mixed bone and nodal disease and 3% had visceral disease at study entry. Among these patients 19% were previously treated with abiraterone alone, 38% with enzalutamide alone, and 44% had abiraterone in combination with enzalutamide, proxalutamide or apalutamide. The phase 2 portion of the study is ongoing and objective tumor responses have been observed including a complete response and partial responses and PSA decreases >50%: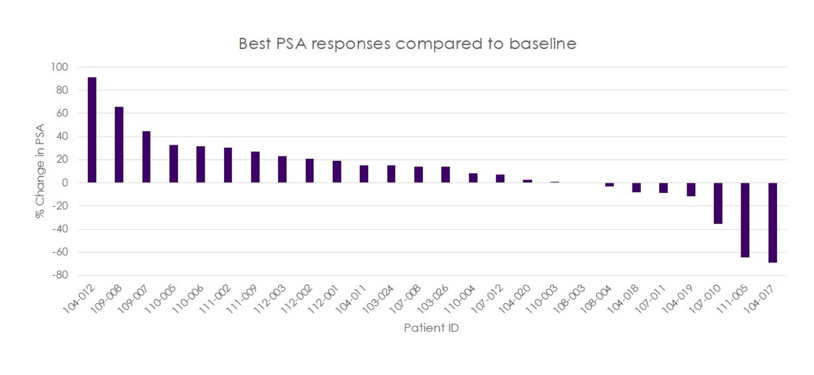
Dr. Markowski concluded with the following take-home messages:
- This phase 1b/2 clinical trial demonstrates that oral daily dosing of sabizabulin (VERU-111) has a favorable safety profile and that chronic dosing is feasible
- The recommended phase 2 dose of 63 mg daily has significant durable antitumor activity, with PSA reductions, objective and durable responses
- These data support a potential prominent role of VERU 111 for the treatment of men with mCRPC who previously failed an androgen receptor targeting agent and prior to the administration of intravenous chemotherapy
- The phase 3 VERACITY clinical trial is currently underway to evaluate the efficacy and safety of sabizabulin versus an alternative androgen receptor targeting agent in men with mCRPC that have failed at least on androgen receptor targeting agent:
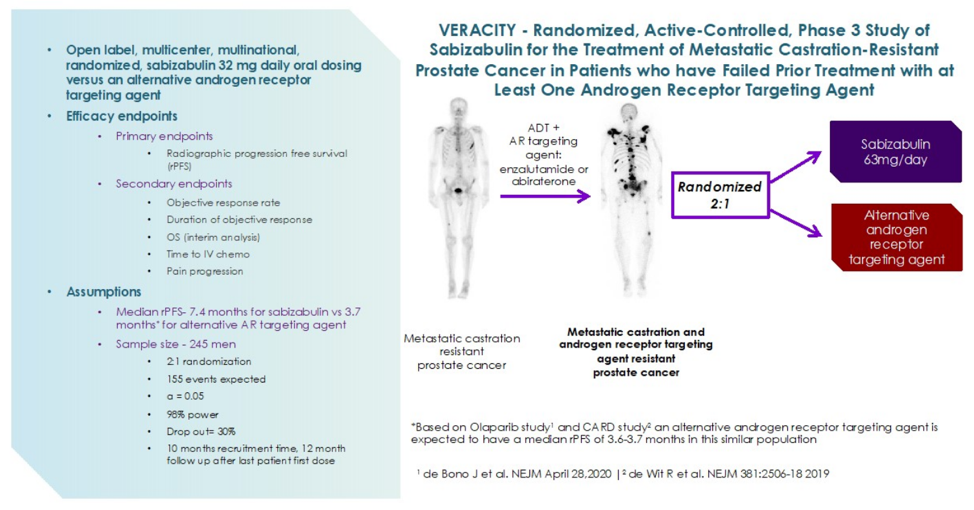
Clinical trial information: NCT03752099
Presented by: Mark C. Markowski, MD, PhD, Assistant Professor of Oncology, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD
Co-Authors: Ronald F. Tutrone, Mario A. Eisenberger, Christopher Michael Pieczonka, Robert H. Getzenberg, Domingo Rodriguez, K. Gary Barnette, Mitchell S. Steiner, Daniel Saltzstein, Emmanuel S. Antonarakis; The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD; Chesapeake Urology, Towson, MD; Associated Medical Professionals of NY PLLC, Syracuse, NY; Veru Inc., Miami, FL; GTx, Inc., Memphis, TN; Urology San Antonio, San Antonio, TX; Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting #ASCO21, June, 4-8, 2021


