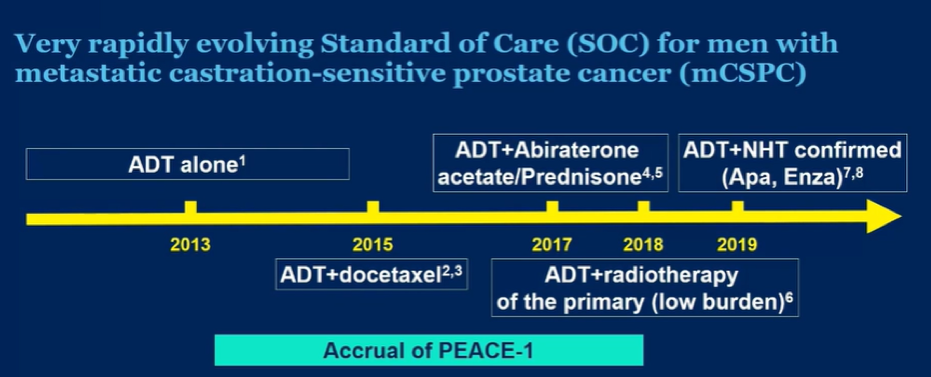
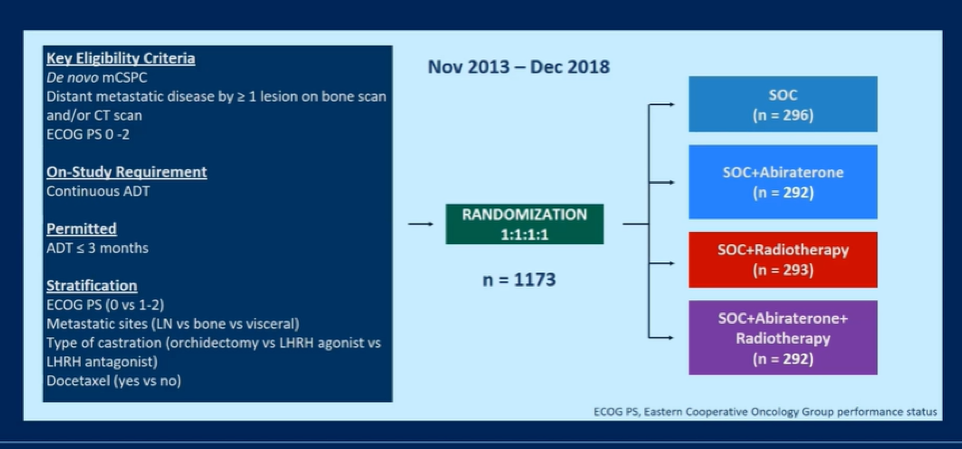
In the PEACE-1 trial (NCT01957436), men with de novo mCSPC were randomized to standard of care (SOC), SOC + abiraterone acetate-prednisone (abiraterone), SOC + radiotherapy, or SOC + abiraterone + radiotherapy. While SOC was initially ADT alone, it evolved as data were published: from Oct 2015 to 2017, docetaxel could be added at the discretion of the treating physician. As of 2017, following the publication of the LATITUDE and STAMPEDE trials, it was mandated that patients receiving ADT + docetaxel. Thus, the authors planned analytically to consider docetaxel as a stratification factor.
PEACE-1 is designed to assess two co-primary endpoints: radiographic progression-free survival (rPFS) and OS with alpha splitting of type I error allocated with 0.1% and 4.9% to rPFS and OS, respectively. As of the current data cut-off, while the required number of rPFS events to achieve 80% power has been reached for the abiraterone comparison, it has not been reached for assessment of the role of radiotherapy.

Analytically, the interaction between abiraterone and radiotherapy was first tested using a Cox model adjusted for stratification factors (performance status, type of castration, metastatic burden, and when applicable, docetaxel). Given the change in SOC during the study, a hierarchical testing was used to test the effect of abiraterone: overall population, then ADT + docetaxel population (standard of care as of 2017 and optional from October 2015 onwards). The authors sought to examine whether there was an interaction between receipt of docetaxel and radiotherapy in order to determine whether arms can be pooled. The authors found no evidence of interaction between radiotherapy and abiraterone on rPFS (p=0.64), allowing for pooling of the two control arms.
Between November 2013 and December 2018, 1173 men were enrolled. The median age at enrollment was 67 years (IQR: 60-72) and the majority of patients (57%) had high volume disease. The SOC was ADT alone for 463 patients and ADT + docetaxel for 710 patients.

With a median follow-up of 42 months for rPFS, the median number of docetaxel cycles was 6 for patients, regardless of treatment arm. For patients who were randomized to abiraterone acetate, median time to abiraterone discontinuation was 31.4 months.
In the overall population, the addition of abiraterone to SOC demonstrated significantly improved rPFS (median rPFS 4.5 years) compared to SOC alone (median rPFS 2.2 years; HR 0.54, 95% CI 0.46-0.64, p < 0.0001). The absolute improvement in median rPFS was 2.3 months.

Among patients where the SOC was ADT + docetaxel, there was a similar benefit to the addition of abiraterone (median rPFS 4.5 years) to SOC alone (median rPFS 2.0 years; HR 0.50, 95% CI 0.40-0.62, p < 0.0001).
Dr. Fizazi then presented results of subgroup analyses, demonstrating that the effect of abiraterone acetate to ADT +/- docetaxel was relatively consistent across subgroups, including those defined by use of radiotherapy, by use of docetaxel, and by metastatic burden.

Dr. Fizazi then presented data on secondary outcomes, including CRPC-free survival and clinical progression-free survival (defined based on radiographic progression, symptomatic progression, or death), demonstrating improvements in rPFS in both the overall study population and among those who received docetaxel as part of the standard of care. When PSA progression was considered an event (bPFS), adding abiraterone again improved outcomes in the overall population (HR 0.40, 95% CI 0.35-0.47, p < 0.0001) and among those where SOC included ADT + docetaxel (HR 0.38, 95% CI 0.31-0.47, p < 0.0001). Thus far, overall survival data is immature.
In terms of toxicity, severe (grade 3-4) adverse events occurring in the first 6 months in more than 5% of patients for whom SOC was ADT + docetaxel included neutropenic fever (4.5% in abiraterone vs 5.4% in control arms), liver toxicity (19.7% in abiraterone vs 13% in control arms), and hypertension (12.2% in abiraterone vs 8.6% in control arms).

Dr. Fizazi concluded that the addition of abiraterone acetate to the standard of care including ADT and docetaxel significantly improves rPFS in men with de novo mCSPC without meaningful differences in short-term toxicity. However, overall survival data are immature.
Presented by: Karim Fizazi, MD, PhD, is a medical oncologist at Gustave Roussy, and a full professor in Oncology at the University of Paris-Saclay in Villejuif, France.
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


