Cohort A1 enrolled patients with post-chemotherapy mCRPC (1–2 prior taxane regimens), ongoing ADT, ≤ 2 prior novel hormonal therapies (abiraterone, enzalutamide, etc) for mCRPC, and no prior PARP inhibitor treatment. Patients received nivolumab 480 mg Q4W + rucaparib 600 mg BID until disease progression/unacceptable toxicity (nivolumab dosing limited to 2 years). The study design for CheckMate 9KD is as follows: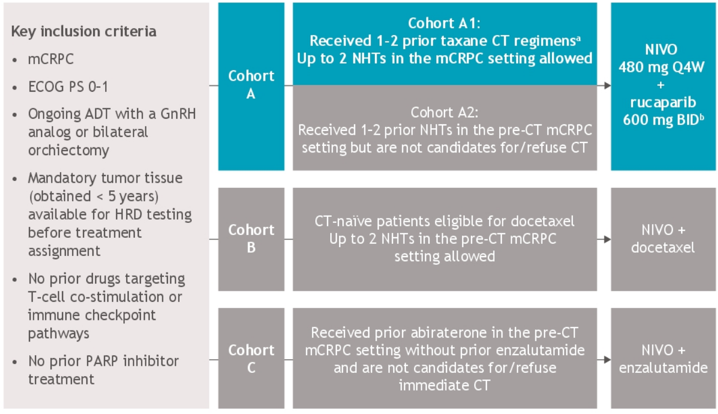
The co-primary endpoints were objective response rate per PCWG3 criteria and PSA response rate (≥ 50% PSA reduction) in all treated patients and patients with homologous recombination deficiency positive tumors, determined before enrollment. Secondary endpoints included radiographic progression-free survival (rPFS), overall survival (OS), and safety.
There were 88 patients treated with nivolumab + rucaparib, with a median age 66 of years (range, 46–85), 34.1% with visceral metastases, and 65.9% had measurable disease. Median follow-up was 11.9 months. Patients had a median of 4.5 nivolumab doses and 4.0 months (range 0.3-17.9) of rucaparib. Among all 58 treated patients with measurable disease at baseline, the confirmed objective response was 10.3% with 6 partial response and no complete responses. Among 84 PSA-evaluable patients, confirmed PSA response rate was 11.9%. Generally, there were better outcomes for homologous recombination deficiency positive versus homologous recombination deficiency negative/not evaluable tumors, including superior objective response rate (17.2% versus 3.4%):
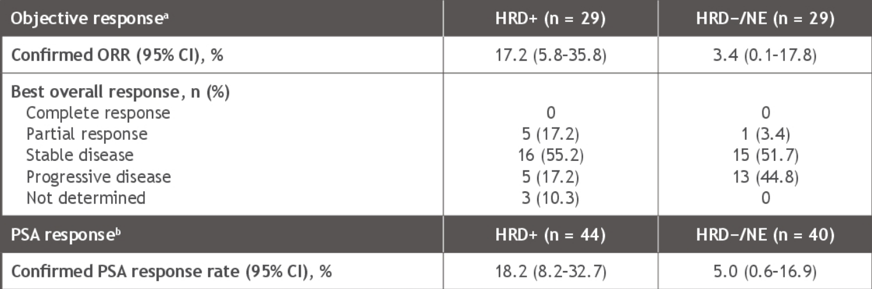
Among 8 patients with baseline measureable disease and BRAC2 alterations, 6 (75%) patients had a >30% reduction in target lesions, with 3 (37.5%) achieving a confirmed objective response. Among 11 PSA-evaluable patients with BRCA2 alterations, 5 (45.5%) patients had a confirmed PSA response. In all 88 treated patients, median rPFS was 4.9 months (95% CI 3.7-5.7)
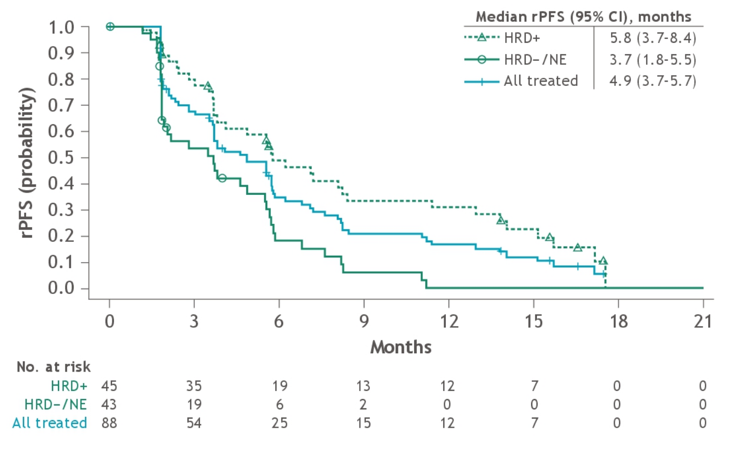
and median OS was 13.9 months (10.4-15.8):

Any-grade treatment-related adverse events occurred in 93.2% of patients, most commonly nausea (40.9%) and fatigue (33.0%). Grade ≥ 3 treatment-related adverse events occurred in 54.5% of patients, most commonly anemia (20.5%) and neutropenia (10.2%). Treatment-related adverse events led to discontinuation in 27.3% of patients, including one patient that had a stroke, considered related to rucaparib by the investigator, after 28 days on rucaparib and 2 nivolumab doses and died 2 months later due to post-thrombolysis hematoma.
Dr. Pachynski concluded this updated analysis of the CheckMate 9KD trial with the following take-home messages:
- Nivolumab + rucaparib is active in patients with homologous recombination deficiency positive post-chemotherapy mCRPC, with noteworthy antitumor activity in those harboring BRCA2 mutations
- However, a lack of study comparator arm and relatively short follow-up for this cohort prevent an adequate assessment of the combination regimen over individual components
- Patients with homologous recombination deficiency negative tumors did not appear to benefit from either drug
- No new safety signals were observed with nivolumab + rucaparib
- Additional biomarker analyses are ongoing, which will further explore potential predictors of treatment benefit with nivolumab + rucaparib among patients with post-chemotherapy mCRPC
Clinical trial information: NCT03338790
Presented by: Russell K. Pachynski, MD, Washington University School of Medicine, St. Louis, MO
Co-Author: Margitta Retz, Jeffrey C. Goh, Mauricio Burotto, Gwenaelle Gravis, Daniel Castellano, Aude Flechon, Stefanie Zschaebitz, David R. Shaffer, Juan Carlos Vazquez Limon, Marc-Oliver Grimm, Steven L. McCune, Neha P. Amin, Jia Li, Xuya Wang, Keziban Unsal-Kacmaz, Fred Saad, Daniel P. Petrylak, Karim Fizazi; Rechts der Isar University Hospital, Technical University of Munich, Munich, Germany; ICON Cancer Centre, Chermside and University of Queensland, St. Lucia, QLD, Australia; Bradford Hill Clinical Research Center, Santiago, Chile; Institut Paoli-Calmettes, Aix-Marseille Université, Marseille, France; Hospital Universitario 12 de Octubre, Madrid, Spain; Centre Léon Bérard, Lyon, France; NCT/Heidelberg University Hospital, Heidelberg, Germany; New York Oncology Hematology, Albany, NY; Instituto Jalisciense de Cancerología, Guadalajara, Mexico; Jena University Hospital, Jena, Germany; Wellstar Health System Inc., Marietta, GA; Bristol Myers Squibb, Princeton, NJ; Centre Hospitalier de l'Université de Montréal, Montréal, QC, Canada; Smilow Cancer Center, Yale School of Medicine, New Haven, CT; Institut Gustave Roussy, University of Paris Saclay, Villejuif, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting #ASCO21, June, 4-8, 2021


