Patients were eligible for KEYNOTE-199 if they had resistance to enzalutamide after prior response. Additionally, prior treatment with abiraterone was allowed. Patients received pembrolizumab 200 mg Q3W for up to 35 cycles + enzalutamide QD until progression, unacceptable toxicity, or withdrawal. The primary end point was objective response rate per RECIST v1.1 by blinded independent central review in cohort 4. Secondary end points were duration of response (cohort 4), and disease control rate, rPFS, OS and safety (in both cohorts). The trial design for KEYNOTE-199 is as follows:
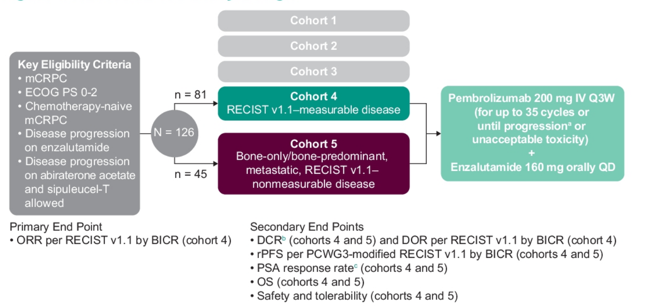
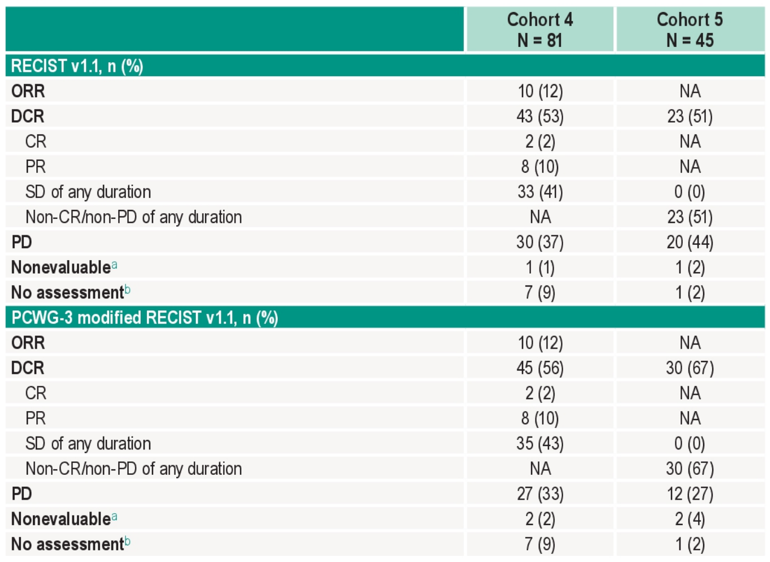
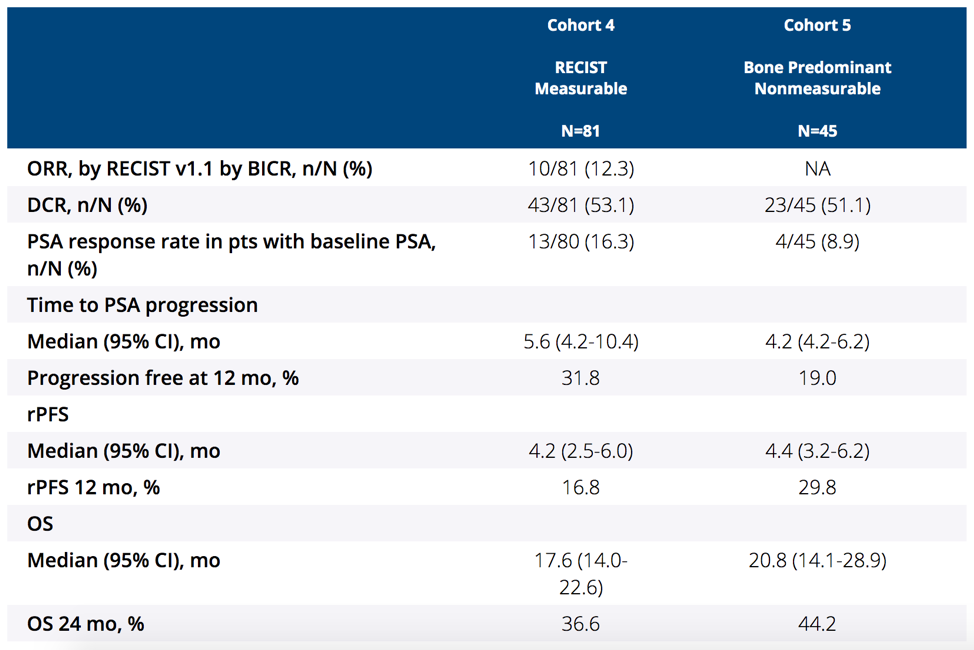
There were 126 patients (cohort 4, n = 81; cohort 5, n = 45) treated, with a median age of 72 years (range 43-92), 32.5% of patients having visceral disease, and 87.3% previously receiving ≥6 months of enzalutamide. There were 121 patients (96.0%) that discontinued therapy, most commonly because of progressive disease. The median time from enrollment to data cutoff was 31.7 months (range: 23.1-37.1) in cohort 4 and 35.5 months (range: 22.9-37.3) in cohort 5. In cohort 4, the confirmed objective response rate was 12.3% (95% CI 6.1-21.5) (2 complete responses, 8 partial responses), with a median duration of response of 8.1 months (range: 2.5+ to 15.2), and 62.5% having a response ≥6 months (Kaplan-Meier estimate):
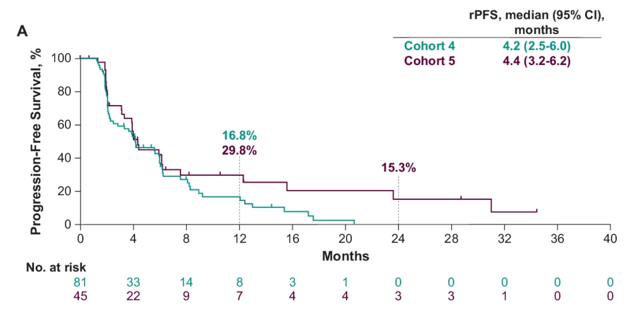
The median rPFS in cohort 4 was 4.2 months (95% CI 2.5-6.0) and for cohort 5 was 4.4 months (95% CI 3.2-6.2):
The median OS in cohort 4 was 17.6 months (95% CI 14.0-22.6) and for cohort 5 was 20.8 months (95% CI 14.1-28.9):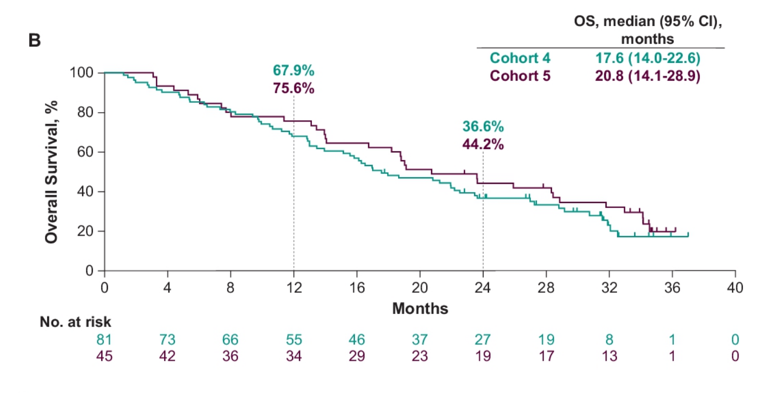
A total of 27.2% and 28.9% of patients in cohort 4 and cohort 5, respectively, experienced grade ≥3 treatment-related adverse events. Two patients in cohort 4 died of immune-related adverse events (Miller Fisher syndrome and myasthenia gravis). Incidence of any-grade (34.1%) and grade 3 or 4 (5.6%) rash, regardless of relatedness to treatment, was higher than previously reported for individual agents but manageable with standard-of-care treatments.
Dr. Graff concluded her presentation of updated data from KEYNOTE-199 with the following summary statements:
- After more than 30 months of follow-up data, pembrolizumab + enzalutamide continued to show modest and durable antitumor activity among patients with mCRPC who became resistant to enzalutamide
- The combination had a manageable safety profile with no new safety signals observed
- The treatment combination is being further evaluated in the ongoing phase 3 KEYNOTE-641 trial (NCT03834493)
Clinical trial information: NCT02787005
Presented by: Julie N Graff, MD, OHSU Knight Cancer Institute, Portland, OR
Co-Author: Scott T. Tagawa, Christopher J. Hoimes, Winald R. Gerritsen, Ulka N. Vaishampayan, Tony Elliott, Clara Hwang, A. J. Ten Tije, Aurelius Omlin, Raymond S. McDermott, Yves Fradet, Deepak Kilari, Cristiano Ferrario, Hiroji Uemura, Cuizhen Niu, Christian Heinrich Poehlein, Ronald De Wit, Charles Schloss, Johann S. De Bono, Emmanuel S. Antonarakis; Weill Cornell Medicine, New York, NY; Duke Cancer Center, Durham, NC; Radboud University Medical Center, Nijmegen, Netherlands; University of Michigan Cancer Center, Detroit, MI; The Christie NHS Foundation Trust, Manchester, United Kingdom; Henry Ford Health System, Detroit, MI; Erasmus MC, Rotterdam, Netherlands; Kantonsspital St. Gallen, St. Gallen, Switzerland; Tallaght University Hospital, Dublin, Ireland; CHU de Québec-Université Laval, Québec City, QC, Canada; Medical College of Wisconsin, Milwaukee, WI; Jewish General Hospital, Montreal, QC, Canada; Yokohama City University Medical Center, Yokohama, Japan; MSD China, Beijing, China; Merck & Co., Inc., Kenilworth, NJ; The Royal Marsden NHS Foundation Trust, London, United Kingdom; Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD


