TALAPRO-1 is a single-arm, open-label, phase 2 study of talazoparib in patients with progressive mCRPC, measurable soft tissue disease, and DNA damage response mutations likely to sensitize to PARP inhibitors (ATM, ATR, BRCA1/2, CHEK2, FANCA, MLH1, MRE11A, NBN, PALB2, RAD51C), who received ≥1 taxane-based chemotherapy and progressed on ≥1 novel hormonal therapy (enzalutamide/abiraterone). The primary objective was confirmed objective response by central independent review, and the assessment of safety included adverse events, incidence of dose modifications and of permanent treatment discontinuation due to adverse events, and clinical laboratory tests.
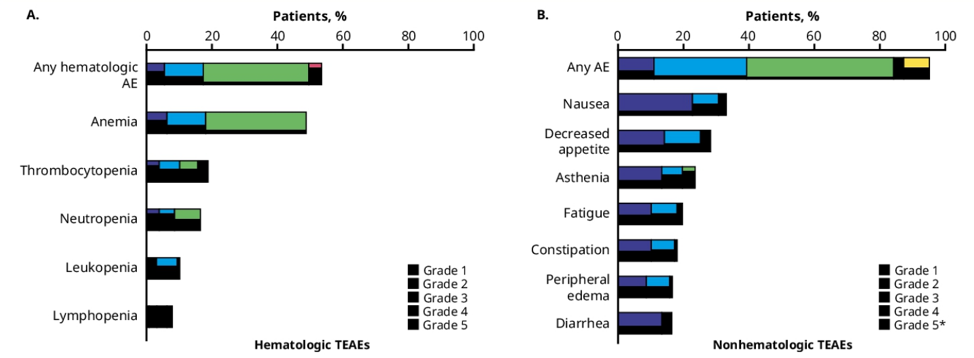
In the talazoparib-treated population (1 mg/daily; n=127), 95.3% (121/127) experienced all-causality adverse events. The most common (≥15%) hematologic adverse events were anemia (any grade, 48.8%; grade 3, 30.7% [no grade 4 events]), thrombocytopenia (all grade, 18.9%; grade 3/4, 8.7%), and neutropenia (all grade, 16.5%, grade 3, 7.9% [no grade 4]). The most common non-hematologic adverse events (≥15%) were nausea (any grade, 33.1%; grade 3/4, 2.4%), decreased appetite (any grade, 28.3%; grade 3/4, 3.1%), and asthenia/fatigue (any grade, 23.6%/19.7%; grade 3/4, 3.9%/1.6%):
Median time from first dose of talazoparib to onset of first episode of grade ≥3 anemia, neutropenia, and thrombocytopenia was 56, 48, and 17 days, respectively. Grade 3 anemia lasted a median of 7 days, grade 3 neutropenia lasted a median of 12 days, grade 3 and grade 4 thrombocytopenia lasted a median of 8 and 11 days, respectively. As follows is the median time from first dose of talazoparib to first grade 3 treatment-emergent hematologic adverse event and duration:
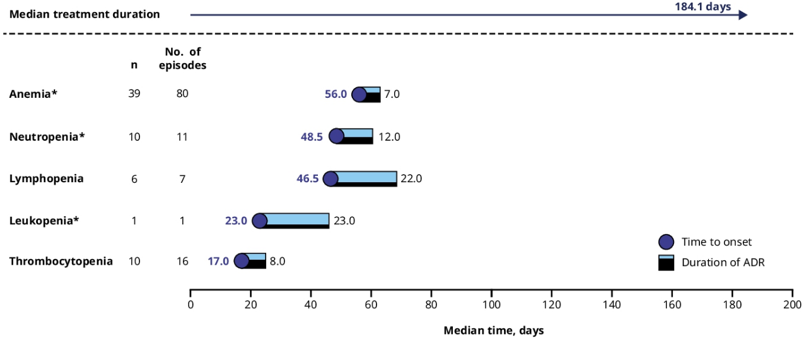
Hematologic adverse events typically occurred during the first 4–5 months of talazoparib treatment and were managed by dose modifications and supportive care. There were 34.6% of patients that received a blood transfusion product, and most transfusions occurred when hemoglobin was between 7.0–10.0 g/dL. Overlapping grade 3/4 hematologic adverse events were infrequent on talazoparib (anemia + neutropenia 4.7%; anemia + thrombocytopenia 5.5%; neutropenia + thrombocytopenia 1.6%). In patients who had anemia, 12.6% also had fatigue; in those with thrombocytopenia, 4.7% had a subsequent bleeding event; in those with neutropenia, 1.6% had an overlapping infection. In the treated population, dose reduction of talazoparib due to all-causality adverse event occurred in 33 patients (26.0%). Treatment discontinuation due to all-causality adverse events was low and occurred in 15 patients (11.8%); the most frequent (≥2 patients) adverse events leading to discontinuation of talazoparib were back pain and platelet count decrease (each, 1.6% [2/127 patients]). There were no treatment-related deaths.
Dr. Mehra concluded this safety analysis of the TALAPRO-1 study with the following summary statements:
- In the TALAPRO-1 study involving men with mCRPC, treatment-emergent adverse events were managed by dose reduction and supportive care
- Adverse event dose reductions most commonly occurred in the first 4-5 months of starting talazoparib
- The most commonly reported all-grade treatment-emergent adverse events were anemia, nausea, decreased appetite, and asthenia
- The demonstrated safety profile, combined with the observed durable antitumor activity, supports further evaluation of talazoparib in advanced prostate cancer. There are 2 ongoing phase 3 trials: TALAPRO-2 (NCT03395197) comparing talazoparib plus enzalutamide with enzalutamide monotherapy as a first-line treatment in men with mCRPC with or without HRR gene alterations, and TALAPRO-3 (NCT04821622) in men with metastatic castration sensitive prostate cancer with HRR alterations
Clinical trial information: NCT03148795
Presented by: Niven Mehra, MD, PhD, Department of Medical Oncology, Radboud University Medical Center, Nijmegen, Netherlands
Co-Author: Karim Fizazi, Johann S. De Bono, Philippe Barthélémy, Tanya B. Dorff, Adam Patrick Stirling, Jean-Pascal H. Machiels, Davide Bimbatti, Deepak Kilari, Herlinde Dumez, Consuelo Buttigliero, Inge M. van Oort, Elena Castro, Hsiang-Chun Chen, Nicola Di Santo, Liza L DeAnnuntis, Cynthia G. Healy, Giorgio V. Scagliotti; Institut Gustave Roussy, University of Paris Saclay, Villejuif, France; The Institute of Cancer Research and Royal Marsden Hospital, London, United Kingdom; Medical Oncology, Institut de Cancérologie Strasbourg Europe, Strasbourg, France; City of Hope Comprehensive Cancer Center, Duarte, CA; ICON Cancer Centre, Queensland, Australia; Cliniques Universitaires Saint-Luc, Brussels, Belgium, and Université Catholique de Louvain, Louvain-La-Neuve, Belgium; Medical Oncology 1 Unit, Department of Oncology, Istituto Oncologico Veneto IOV IRCCS, Padua, Italy; Division of Hematology and Oncology, Medical College of Wisconsin, Milwaukee, WI; Department of General Medical Oncology, University Hospitals Leuven, Leuven Cancer Institute, and Laboratory of Experimental Oncology, Department of Oncology, KU Leuven, Leuven, Belgium; Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Orbassano, Turin, Italy; Department of Urology, Radboud University Medical Center, Nijmegen, Netherlands; Hospital Universitario Virgen de la Victoria, Instituto de Investigación Biomédica de Málaga (IBIMA), Málaga, Spain; Pfizer Inc., La Jolla, CA; Pfizer Inc., Durham, NC; Pfizer Inc., Collegeville, PA


