(UroToday.com) Beginning with the introduction of docetaxel for metastatic castration resistant prostate cancer (mCRPC) in 2004, there has been a dramatic and rapid proliferation of systemic therapy options in advanced prostate cancer. Following developments in the mCRPC disease space, many of the same treatments have now been adopted in patients with metastatic castration sensitive disease (mCSPC). Since 2015, the addition of docetaxel, novel hormonal therapies (including abiraterone acetate, enzalutamide, and apalutmide), and local prostate-directed radiotherapy (in those with low-volume metastases) have proven benefit in patients with mCSPC. Tak is an oral selective nonsteroidal 17, 20-lyase inhibitor that blocks the synthesis of gonadal and adrenal androgens. It has previously been assessed in metastatic castration-resistant disease.

In the Prostate, Testicular, and Penile Oral Abstract Session at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Dr. Agarwal presented the first results of the SWOG S1216 trial (NCT01809691) assessing TAK-700 in patients with metastatic hormone-sensitive prostate cancer.
The authors accrued patients with newly diagnosed mHSPC with a Zubrod performance status of 0-2 and a PSA of ≥ 2 ng/ml and randomized them in a 1:1 fashion to ADT + Tak (300 mg twice daily) or ADT + bicaluamide (50 mg daily). Randomization was stratified by PS (0-1 vs ≥2), extent of disease (minimal vs extensive), and receipt of ADT prior to registration (yes vs no).

The primary endpoint of the trial was overall survival (OS) with secondary endpoints including progression free survival (PFS; based on PSA, imaging or clinical progression), PSA at 7 months (≤0.2 vs 0.2 < PSA; ≤-4 vs. > 4 ng/ml) and adverse event (AE) profile.
The authors planned to accrue 1,186 eligible patients over 2.75 years, with a further 3 additional years of follow-up. Doing so would provide 90% power to determine a 33% improvement in OS from 54 to 72 months (1-sided α = 0.025). Final analysis was planned after 523 deaths using a 1-sided α = 0.022 to account for interim analyses.
The authors randomized 1313 patients between March 2013 and July 2017 of whom 32 were ineligible and 2 withdrew consent, leaving 1279 patients in the intention-to-treat (ITT) analysis. The median age of included patients was 68 years and 10% of subjects were Black. At enrollment, the median PSA was 30 ng/mL (range 2-6710) and 49% of patients had extensive disease. Notably, Dr. Agarwal highlighted that Black men comprised 10% of this study cohort.

Dr. Agarwal presented that approximately 51% of included patients had so-called “minimal disease severity”. Additionally, he noted that approximately one-quarter of patients had previously received radical prostatectomy. Only 5% of patients received bisphosphonates at study entry.
As of the data cut-off, 192 (30.1%) of patients receiving TAK-700 and 100 (15.6%) of patients receiving bicalutamide remained on therapy. Over a median follow-up of 4.9 years, median overall survival was longer among those who received ADT + Tak (81.1 months) compared to ADT + bicalutamide (70.2 months) though this did not meet the pre-specified alpha threshold of 0.022 (hazard ratio 0.86, 95% CI 0.72-1.02, p=0.04).

Subgroup analyses demonstrated generally comparable results across a variety of baseline characteristics. There was a suggestion of less benefit among men who received androgen deprivation therapy prior to registration.
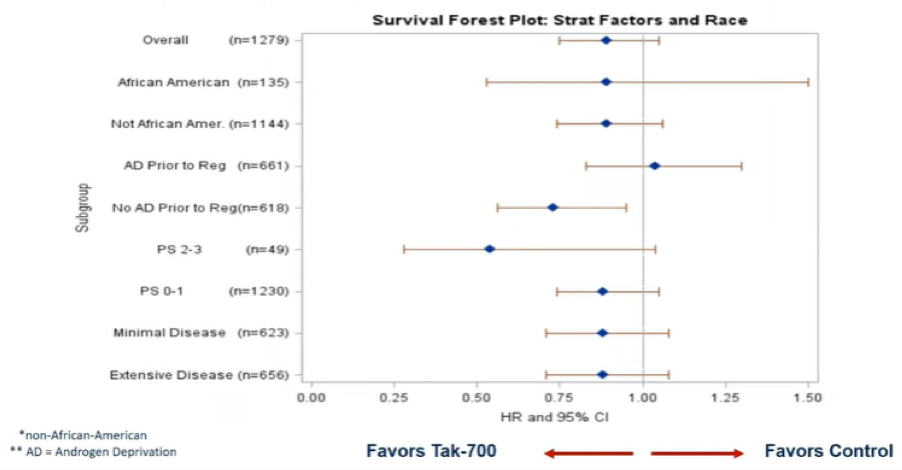
In contrast to the non-significant benefits in overall survival, improvements in progression-free survival (median 47.6 months vs 23.0 months) were statistically significant (hazard ratio 0.58, 95% CI 0.51-0.67, p<0.0001).

Secondary endpoints favored the use of TAK-700, with PSA response rates significantly improved (58.3% vs 44.0% for PSA ≤0.2 ng/mL, p<0.0001) for men in this treatment arm.
As may be expected, grade 3-4 adverse events were more common among patients receiving Tak (43%) than bicalutamide (14%), including hypertension (20% vs. 5%) and fatigue (5% vs. 2%). Additionally, grade 5 adverse event occurred in 5 patients treated with Tak and in 2 patients treated with bicalutamide.

As median overall survival was substantially longer among men in the control arm than expected, the authors performed post hoc analyses according to the use of subsequent life-prolonging therapy. Among men randomized to TAK-700, 61.3% of patients received subsequent post-protocol life-prolonging therapy while rates were somewhat higher among those randomized to bicalutamide (77.4%). Dr. Agarwal then provided a comparison of these results to contemporary phase III trials in this disease space. He emphasized that survival in the comparison of this SWOG S1216 trial is substantially longer among patients in the control arm, compared to previous studies, highlighting that the overall quality of care patients are receiving (including in subsequent stages of disease) has improved over time.

The authors conclude that, despite clinically meaningful improvement in various outcome measures with Tak + ADT over ADT + bicalutamide in men with mHSPC, the improvement in OS did not meet the pre-specified criteria for statistical significance. He further highlighted that this study is strengthened by the enrollment of patients from both academic and community centers including higher than prior enrollment of African American men.
Presented by: Neeraj Agarwal, MD, Professor in the Division of Oncology, Department of Medicine, at the Huntsman Cancer Institute (HCI) at the University of Utah School of Medicine. He is the Huntsman Cancer Institute (HCI) Presidential Endowed Chair of Cancer Research, and the Director of the Genitourinary Oncology Program, Dr. Agarwal also serves as the physician-scientist and senior director of clinical research innovation at HCI, Salt Lake City, Utah
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting #ASCO21, June, 4-8, 2021


