
Based on clinical experience combining gamma ray radiation therapy with inhibition of hormonal signaling, the ERA-223 (NCT02043678) trial tested the hypothesis that combining the alpha emitting radiopharmaceutical radium-223 with the androgen signaling pathway inhibitor abiraterone would improve clinical outcomes in mCRPC. The schema of the trial is shown below.

In 2017, the independent data monitoring committee for the trial recommended unblinding after more fractures and deaths were noted in the abiraterone plus radium-223 arm. Of patients on the trial, 60% were not receiving bone protection agents, and a post-hoc analysis suggested that these agents decreased the rate of fractures in both arms.
The PEACE-3 trial was designed to test a similar question as ERA-223, using the androgen recept (AR) inhibitor enzalutamide instead of abiraterone. The trial schema is shown below.

After the ERA-223 data on increased fracture risk became known, the PEACE-3 trial was amended to include bone protecting agents in both arms. Preliminary data suggested this effectively reduced fracture rates, and in this presentation, Dr. Gillessen presented updated data on the risk of bone fractures for patients enrolled on PEACE-3. The number of patients treated before and after the safety amendment in each arm are shown below.
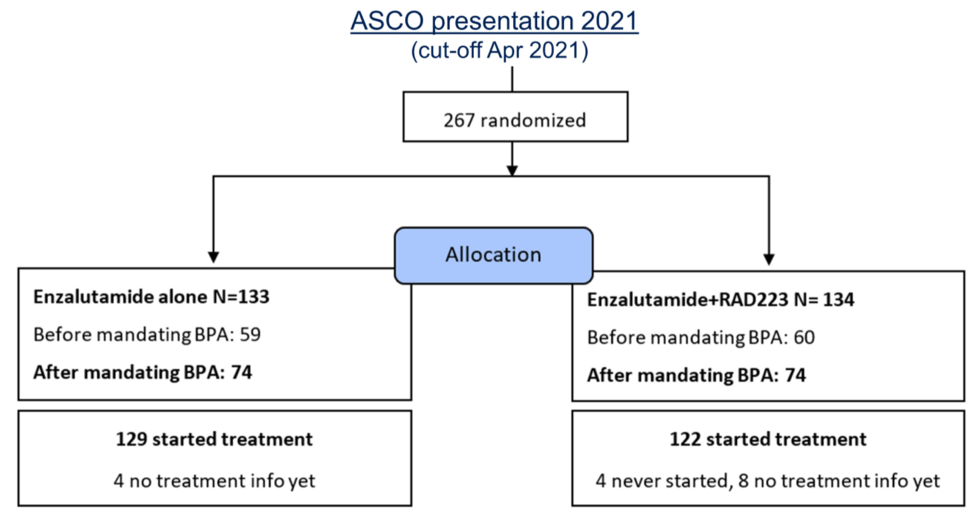
Prior to the safety update, only 46.1% of patients on trial were taking a bone-protecting agent, but afterward, 96.3% of patients were doing so. At one year of treatment, patients not taking bone-protecting agents had a fracture risk of 15.6% in the enzalutamide only arm and 37.1% for enzalutamide plus radium-223. After the initiation of a bone-protecting agent, the fracture risk in the enzalutamide arm decreased to 2.6% and to 2.7% in the enzalutamide plus radium-223 arm. Dr. Gillessen concluded that these data confirm the importance of bone-protecting agents when treating mCRPC with bone metastatic disease to prevent complications.
Presented by: Silke Gillessen, MD, senior consultant in the Medical Oncology-Hematology Department at the Kantonsspital St. Gallen, St Gallen, Switzerland
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


