
Regarding the first presentation, data on radiographic progression free survival (rPFS) with the addition of abiraterone in de novo mHSPC from the PEACE-1 study, Dr. Horvath noted that the standard of care control arms evolved over the course of this trial from ADT alone to ADT +/- docetaxel to ADT + docetaxel given if a patient was randomized to not receive abiraterone, then they could receive the unethical treatment option of ADT alone. A total of 1173 patients were enrolled, including 57% having high volume disease and 60% received docetaxel. As there was no interaction for the rPFS endpoint between abiraterone and the administration of radiotherapy, all patients receiving ADT and docetaxel could be included in the overall analysis, stratified by whether they received abiraterone. The results of the trial are shown below and are notable for a statistically significant improvement in rPFS with abiraterone without excessive new toxicity.

Somewhat confounding the interpretation of the trial is the moving target of what constitutes standard of care in de novo mHSPC. For high volume disease by the CHAARTED definition, the treatment standard options are ADT + systemic treatment, the latter of which can be docetaxel, abiraterone, enzalutamide or apalutamide. For low volume disease, docetaxel is not commonly used based on data from CHAARTED and GETUG-AFU-16, though the STAMPEDE dataset which has almost all patients with de novo metastatic disease suggested a benefit for docetaxel.
Dr. Horvath noted that this study represents the largest study so far to potentially address the use of therapy triplets (ADT, chemotherapy, androgen pathway inhibitor) in metastatic CSPC, and met its primary endpoint of prolonged rPFS. However, rPFS is not a proven surrogate for overall survival, though this may change pending results from the STOPCAP group. Dr. Horvath then compared the PEACE-1 trial to other similar trials, specifically ENZAMET, in which some patients did receive triplet therapy. While ENZAMET met its primary endpoint of improving overall survival, it only showed rPFS benefit but not overall survival for triplet therapy. Longer data follow-up from ENZAMET and PEACE-1 are required to further delineate the true advantage of triplet treatment.
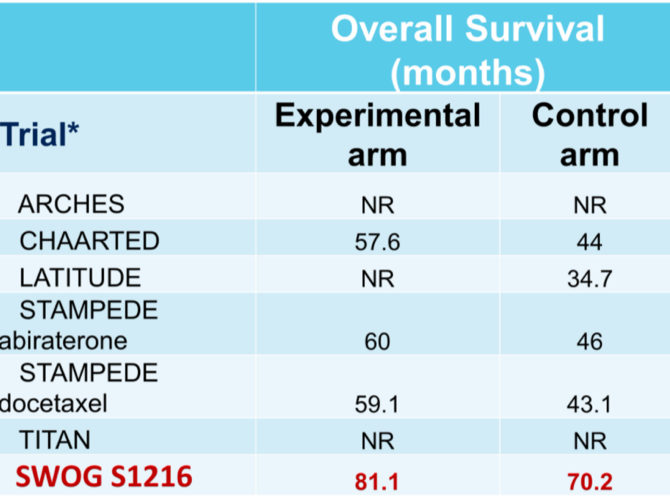
She then turned her attention to SWOG1216, looking at whether TAK-700 improved overall survival in mHSPC. This study did not meet its endpoint. However, this may be partly due to the long overall survival in the control arm, which is the highest of the contemporary trials in this space.
Finally, Dr. Horvath summarized the discussion around the safety data from the PEACE-3, specifically whether the use of bone protecting agents overall reduces rates of fracture in CRPC. The answer to this question using either zoledronic acid or denosumab is promising, and the incidence of fracture in each arm is shown below.

Dr. Horvath stated that this trial illustrated the value of the internal data monitoring committees (IDMC), and successfully decreased the risk of fractures in mCRPC. On balance, the risk of fracture was reduced by 13%, compared to less than 5% for risk of osteonecrosis of the jaw for denosumab and less than 2% for bisphosphonates.
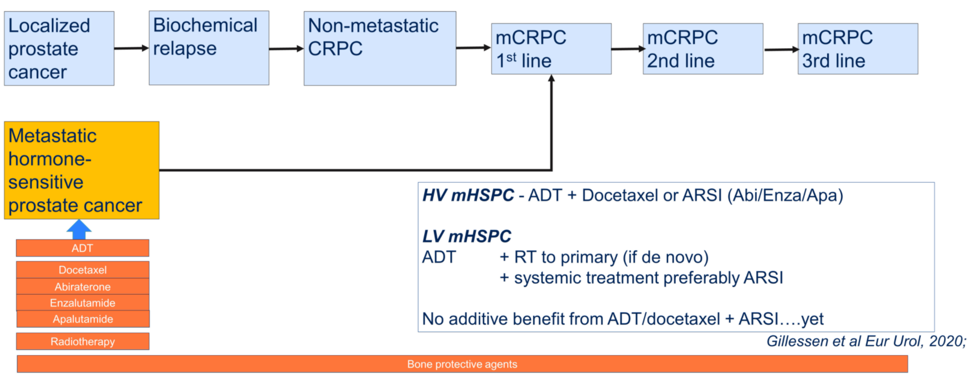
After summarizing the three presentations, Dr. Horvath then posed the question of whether we can define the standard of therapy for first-line metastatic hormone-sensitive prostate cancer, and showed the graphic below? She stated that she does not believe these data change recommendations from the 2019 APCCC consensus conference, but that she looks forward to the next conference to see if this is the case.
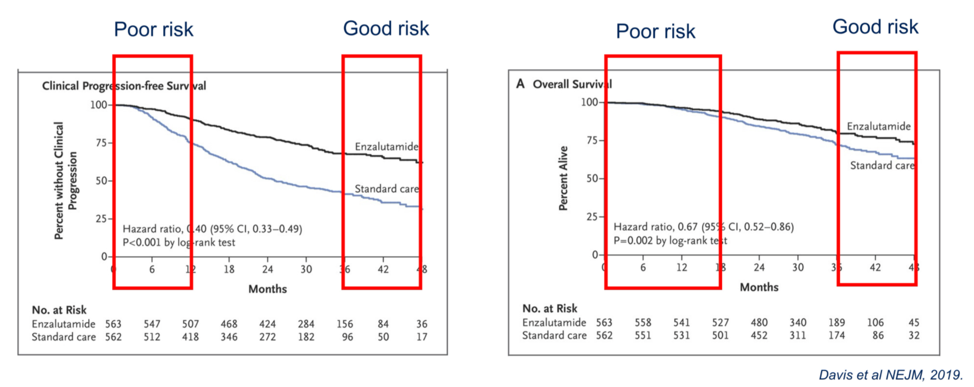
Overall, how do we start to decide who needs more intensive or triplet therapy in metastatic hormone sensitive prostate cancer? Looking at the progression free and overall survival curves from the ENZAMET study, Dr. Horvath identified a subset of patients that do poorly initially with progressive disease and death, whereas there is a subset of patients with good prognosis who do well either with ADT alone or the addition of enzalutamide and/or docetaxel.
To address this question, the STOPCaP and ICECaP working groups are identifying clinical stratification groups in a manner analogous to risk stratification in localized prostate cancer. The different clinical parameters that are being explored by these groups are shown below.

Finally, further elucidation of the biology of different groups of mHSPC to identify correlates of different clinical behavior may be helpful. Patient samples from some of the major practice changing trials in mHSPC are currently being subjected to biomarker analysis, which may optimize patient selection for different treatment strategies.
Presented by: Lisa Horvath, PhD, MBBS, FRACP, Professor of Medical Oncology, Chris O’Brien Lifehouse, University of Sydney, Australia
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


