The first abstract discussed by Dr. Gilligan was by Dr. Darren Feldman “Immunity to childhood vaccines following high dose chemotherapy and autologous stem cell transplantation for germ cell tumors with comparison to Hodgkin lymphoma”. In this study, among 80 patients with germ cell tumor (median age 30, 84% nonseminoma), 91% received 3 sequential transplants and 68 had repeat titers at ≥6 months. Immunity at baseline was >95% for Diphtheria, Tetanus and Polio and 89% for Varicella Zoster but lower for Measles (74%), Mumps (85%), and Rubella (83%):

The major take-away messages for Dr. Gilligan were that (i) adult men with germ cell tumors do not lose immunity to child vaccines after high-dose chemotherapy, and (ii) up to 31% of these men lack immunity to MMR at baseline. However, in a study of NBA and MLB athletes, 30% lacked immunity to at least one of the three viruses, such that MMR is highly effective at inducing immunity but the effect wanes over time. Questions for future research are:
- Do disseminated germ cell tumor patients previously treated with chemotherapy have low MMR immunity?
- Should we use their engagement with the healthcare system as an opportunity to encourage vaccination for inadequately immunized individuals?
The second study discussed was by Dr. Shirin Ardeshirrouhanifard “Hearing loss after cisplatin-based chemotherapy: Patient-reported outcomes versus audiometric assessments.” Cisplatin is associated with dose-dependent bilateral sensorineural hearing loss, with a 3.2-dB decline in the overall hearing threshold for every 100 mg/m2 increase in cumulative dose. In this study of 1,410 testicular cancer survivors, 34.8% of testicular cancer survivors self-reported hearing loss, while 77.8% had audiometrically-defined hearing loss. Among testicular cancer survivors without tinnitus, those with audiometrically-defined hearing loss at only extended high frequencies (10-12 kHz) (17.8%) or at both extended high frequencies and standard frequencies (0.25-8 kHz) (23.4%) were significantly more likely to self-report hearing loss than those with no audiometrically-defined hearing loss (8.1%) (OR 2.48, 95% CI 1.31-4.68 and OR 3.49, 95% CI 1.89-6.44, respectively). Risk factors for cisplatin-induced hearing loss included older age, higher cumulative cisplatin dose, hypertension, and lower educational achievement. Dr. Gilligan notes that sodium thiosulfate has been used for preventing ototoxicity, noting a 48% lower incidence of hearing loss when combined with cisplatin chemotherapy versus chemotherapy alone (relative risk 0.52, 95% CI 0.33-0.81) [1]. Questions for future research include:
- Is there any way to reduce ototoxicity from cisplatin? Larger scale validation of sodium thiosulfate
- What is the most effective strategy for reducing cisplatin exposure for these patients?
The third study discussed was by Dr. Jennifer King “Surveillance after complete response in patients with metastatic NSGCT”. This patient population is one of men with metastatic testis cancer who have retroperitoneal adenopathy prior to chemotherapy and a complete response to chemotherapy. The dilemma is whether we should recommend a post-chemotherapy RPLND for these patients. In a 2003 Norwegian study assessing 62 men with post-chemotherapy retroperitoneal nodes <= 10 mm who underwent RPLND, 8% had residual cancer, 18% had teratoma and 61% had necrosis/fibrosis [2]. In a 2010 study from the British Columbia Cancer Agency and the Oregon Testis Cancer Program, 161 patients received first-line chemotherapy with resolution of tumor markers and no residual masses >1 cm [3]. Overall, 10 patients (6%) relapsed, with 8/10 being teratoma and a disease-specific survival of 100% over a median follow-up of 64 months (range: 4-105). The current study was the Indiana experience, including 388 patients that met eligibility and were included in this analysis. With a median follow-up of 3.9 years, 34 patients (8.8%) had disease progression. At most recent follow-up, 363 (93.6%) patients were alive with no evidence of disease, and 10 patients (2.6%) died of their disease. The estimated 2-yr PFS was 90.1% (95% CI 86.2-93%) and 2-yr OS was 97.8% (95% CI 95.2-99%). The estimated 2-yr PFS by IGCCCG risk category was 90.4% for good versus 90.4% for intermediate versus 86.5% for poor-risk (p = 0.23):

The estimated 2-yr OS was 98.6% for good versus 95.5% for intermediate versus 92.9% for poor risk disease respectively (p = 0.002):

For the 34 patients who progressed on surveillance, 16 (4%) progressed in the retroperitoneum only. There were 3 patients that had malignant transformation of teratoma to PNET, adenocarcinoma, or other elements. Furthermore, 11 of progressed patients were treated with surgery, 12 were treated with salvage chemo, and 11 were treated with surgery + chemotherapy. At most recent follow-up, 21 of progressed patients showed no evidence of disease, 10 had died of disease, and 3 were lost to follow-up.
Dr. Gilligan notes that if we combine the two North American studies ([3] + the Indiana experience) we have 549 men with no residual mass >1 cm, 44 relapses, and 10 deaths, and thus the questions remain: 1) Should we recommend 549 post-chemotherapy RPLNDs to try to prevent 44 relapses and 10 deaths? 2) How many of these deaths would be prevented by RPLND? 3) How can we identify the 10% of patients destined to relapse? One possibility is the utilization of micro-RNA to help decide who needs a post-chemotherapy RPLND or more chemotherapy. miRNA levels are strongly associated with viable residual cancer, but miRNA levels are not yet accurate enough for detecting teratoma.
The fourth study discussed was by Dr. Andreas Wibmer “Effect of pretreatment central adiposity on the cardiometabolic risk of male germ cell tumor survivors after cisplatin-based chemotherapy”. Chemotherapy for testis cancer is associated with an increased risk of death from cardiovascular disease, although recent studies report that risk is mainly in the 12 months after starting chemotherapy. Dr. Gilligan notes that the mechanism is likely multifactorial, which likely includes metabolic syndrome components (dyslipidemia, hypertension), endothelial dysfunction, and hypercoagulability. The hypothesis for this study was that visceral/central adiposity is associated with higher risk of cardiometabolic risk in male germ cell tumor survivors. The cardiometabolic risk metrics used in this study were (i) new hypertension, dyslipidemia or type 2 diabetes, and (ii) increased Framingham cardiovascular risk score. Of note, this study did not use cardiovascular events as an endpoint. Among 455 men in this study, the median VAT/SAT ratio at baseline CT was 0.49 and positively associated with higher post-chemotherapy Framingham risk scores after adjustment for age, BMI, and blood pressure measurements at chemotherapy start, as well as post-therapy follow-up time (adjusted β-estimate: 1.36, 95% CI 1.15, 1.59). A higher VAT/SAT ratio also inferred a higher likelihood of new-onset cardiometabolic disease in patients with BMI ≥30 kg/m2 (age-adjusted HR 3.12, 95%CI 1.00, 9.71), but not in those with BMI < 30 kg/m2 (HR 2.08, 95% CI 0.59-7.32).
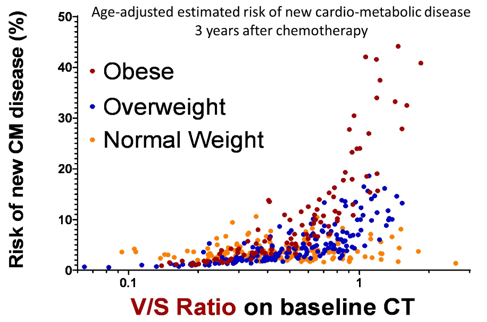
Dr. Gilligan’s take-home messages from this study were that (i) central adiposity is associated with increased risk of new-onset cardiometabolic disease, and (ii) men who gain weight after chemotherapy for germ cell tumors tend to add fat in the visceral compartment. Questions for future research for this study include:
- What is the role of chemotherapy in this risk?
- Is this risk modifiable?
Dr. Gilligan summarized his discussion of these four abstracts with the following points:
- Men with disseminated germ cell tumors do not lose immunity to childhood vaccines after high-dose chemotherapy but nearly a third of men have inadequate immunity prior to high-dose chemotherapy
- Testis cancer survivors without tinnitus underestimate their hearing loss
- Men with disseminated germ cell tumors who have normal serum tumor markers and no residual masses larger than 10 mm can be safely observed without undergoing RPLND
- Central fat distribution in testis cancer survivors is associated with an increased risk of developing cardiometabolic disease
Presented by: Timothy D. Gilligan, MD, Cleveland Clinic, Cleveland, OH
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
References:
- Brock, PR, Maibach R, Childs M, et al. Sodium Thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med. 2018 Jun 21;378(25):2376-2385.
- Oldenburg J, Alfsen GC, Lien HH, et al. Postchemotherapy retroperitoneal surgery remains necessary in patients with nonseminomatous testicular cancer and minimal residual tumor masses. J Clin Oncol. 2003 Sep 1;21(17):3310-3317.
- Kollmannsberger C, Daneshmand S, So A, et al. Management of disseminated nonseminomatous germ cell tumors with risk-based chemotherapy followed by response-guided postchemotherapy surgery. J Clin Oncol. 2010 Feb 1;28(4):537-542.


