(UroToday.com) At the 2022 American Society of Clinical Oncology Annual Meeting held in Chicago and virtually, the poster session focused on Kidney and Bladder cancers on Saturday afternoon included a trials in progression presentation from Dr. Neal D. Shore who described the rationale and design of the CREST Study Cohort B, examining sasanlimab as monotherapy in patients with bacillus Calmette-Guérin (BCG) unresponsive high-risk, non-muscle invasive bladder cancer (NMIBC).
The majority of patients with newly diagnosed bladder cancer will have NMIBC. Among these, most will have low-grade, low-risk disease. However, those who are found to have higher risk disease at the time of TURBT are recommended to receive adjuvant intravesical Bacillus Calmette-Guérin (BCG) on the basis of decreased risks of recurrence. While BCG is efficacious, a significant proportion of patients with develop BCG refractory for whom the standard of care is radical cystectomy. Many patients may be unwilling or unable to undergo cystectomy, and as such, there is a significant unmet need to identify alternative intravesical therapies to minimize the risk of recurrence and progression.
In a phase 1 study of sasanlimab (PF-06801591), a monoclonal antibody to programmed cell death protein 1 (PD-1), administered subcutaneously at 300 mg every 4 weeks, there was evidence of an acceptable safety profile and promising clinical activity, will allowing subcutaneous administration. To build on this, the CREST Study (NCT04165317) Cohort B aims to evaluate sasanlimab administered subcutaneously in patients with BCG-unresponsive NMIBC in a phase III study.
The authors have designed CREST Study Cohort B as a non-randomized, multicenter, multinational, open-label, phase 3 study. The authors plan to enroll ̃160 patients with histologically confirmed BCG-unresponsive, high-risk, non-muscle invasive transitional cell carcinoma of the bladder urothelium (high-grade Ta or T1 tumor, or carcinoma in situ [CIS]) in 2 separate Cohorts, B1 and B2 (̃110 and ̃50 patients, respectively).

Cohort B will be divided into two. Cohort B1 will enroll patients with persistent or recurrent CIS with or without concomitant recurrent high-grade Ta/T1 disease, within 12 months of completing adequate BCG therapy. Cohort B2 will enroll patients with recurrent high-grade Ta/T1 disease within 6 months of completing adequate BCG therapy.
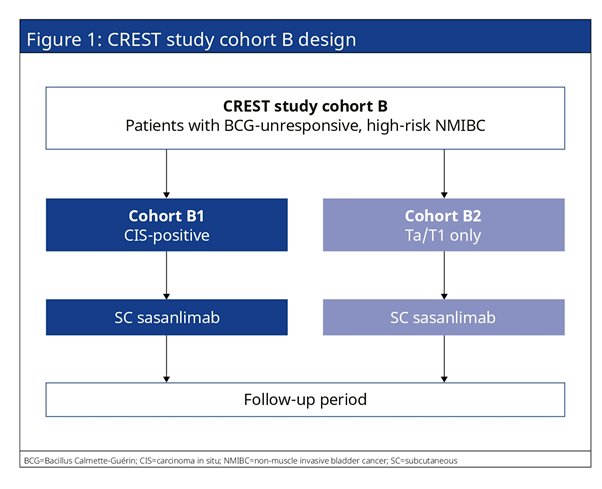
All patients will receive subcutaneous sasanlimab monotherapy. Patients will be followed at regular intervals by cystoscopy, urine cytology, biopsy, and imaging to assess for efficacy. The primary endpoint is complete response (CR) and event-free survival (EFS) for Cohorts B1 and B2. Secondary endpoints include duration of complete response (Cohort B1 only), EFS (Cohort B1 only), overall survival, time to cystectomy, safety, health-related quality of life, pharmacokinetic parameters, PD-L1 expression, and incidence of anti-drug antibodies.
This study will initially open for recruitment in Canada and the United States of America, with subsequent sites in Asia, Australia, and Europe.
Presented by: Neal Shore, MD, FACS, is the Medical Director of the Carolina Urologic Research Center. He practices with Atlantic Urology Clinics in Myrtle Beach, South Carolina


