(UroToday.com) The 2022 ASCO annual meeting featured a session on kidney and bladder cancer, including a presentation by Dr. Gopa Iyer discussing the association of DNA damage response gene mutations and response to neoadjuvant cisplatin-based chemotherapy in MIBC patients enrolled in SWOG S1314. Neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy is a standard of care treatment for patients with MIBC. Mutations within the nucleotide excision repair DNA helicase ERCC2, and several other DDR genes, have been implicated in cisplatin sensitivity in MIBC. Furthermore, DDR gene mutations, including within ERCC2, have been associated with higher pathologic downstaging (< pT2) and complete response (pT0) at radical cystectomy and improved overall survival in retrospective series. SWOG S1314 randomized patients to one of 2 chemotherapy regimens (dose-dense MVAC or gemcitabine/cisplatin) followed by radical cystectomy. Dr. Iyer and colleagues sought to correlate ERCC2 and other DDR gene mutations with response and survival in MIBC patients enrolled in the prospective SWOG S1314 trial.
For this study, tumor and matched germline DNA from evaluable patients enrolled onto S1314 underwent exon capture sequencing of 505 cancer-associated genes (MSK-IMPACT). Both deleterious mutations and any mutations in 9 DDR genes (ERCC2, ERCC5, BRCA1, BRCA2, RECQL4, ATM, ATR, RAD51C, FANCC) were correlated with clinical outcomes. The prespecified analyses included the association of mutations with < pT2 and pT0 by logistic regression analysis and with progression-free survival (PFS) and overall survival (OS) by Cox proportional hazards regression.
There were 179 patients, with a median 61 years of age, 85% of which were male, 87% of which were white, and 87% with clinical stage T2. These patients received >2 cycles of chemotherapy, were evaluable for pathologic response, and were included in the analysis. Overall, the pT0 rate was 28% and < pT2 was 41%. Deleterious mutations in ERCC2 were detected in 26 (14%) patients, followed by ATM (n = 12, 7%), ATR (n = 3), and BRCA2 (n = 2). ERCC2 mutations were associated with statistically significantly higher pathologic responses with a 54% pT0 rate and 62% downstaging rate. Patients with any deleterious mutations had higher pathologic response rates (51% pT0, 56% < pT2) and better PFS with a median follow-up of 53 months. There was also a non-significant trend towards improved overall survival:
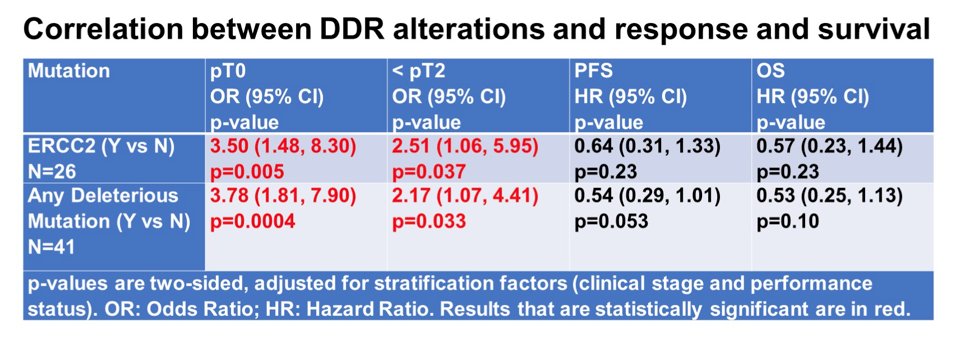
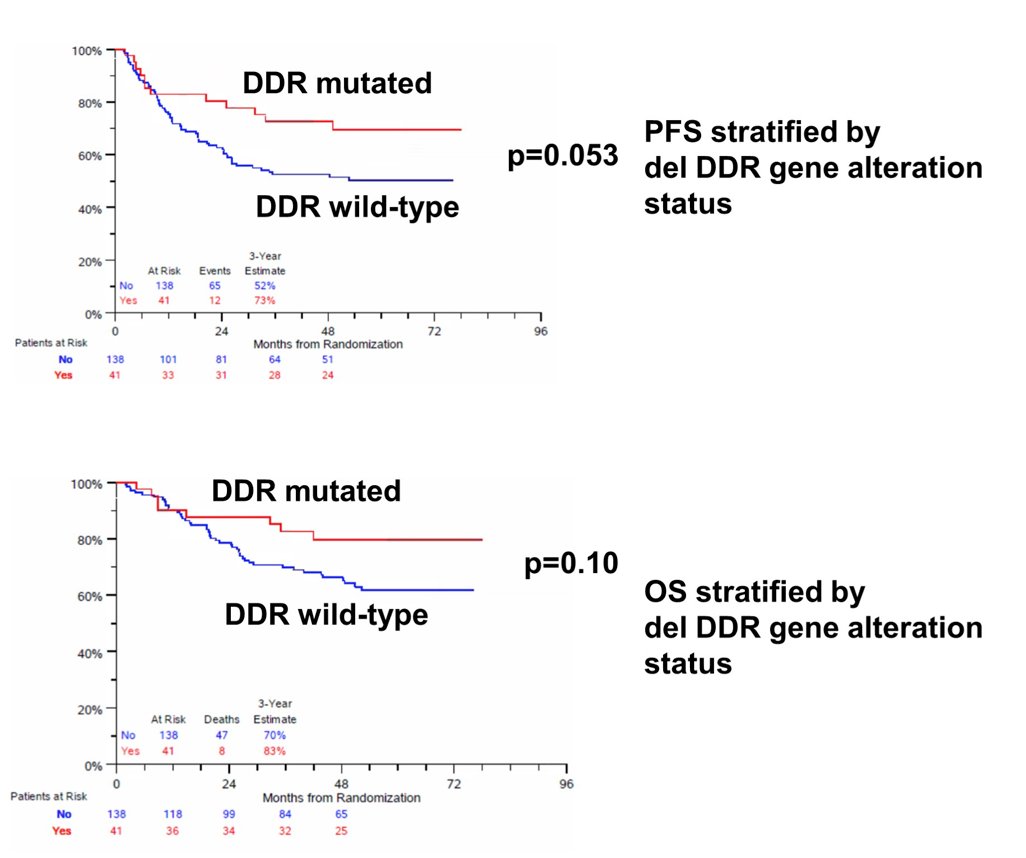
The Kaplan Meier curves for PFS and OS stratified by deleterious DDR gene alteration status are as follows:
Dr. Iyer concluded this presentation by discussing the association of DNA damage response gene mutations and response to neoadjuvant cisplatin-based chemotherapy in MIBC patients enrolled onto SWOG S1314 with the following take-home messages:
- In patients managed with neoadjuvant chemotherapy and radical cystectomy on S1314, both ERCC2 mutations and deleterious DDR gene mutations correlated with pathologic response (<pT2 and pT0)
- Any deleterious DDR gene mutation was associated with improved PFS
- These results are in line with retrospective analyses displaying a correlation between DDR gene mutations and neoadjuvant chemosensitivity in MIBC
- Ongoing trials are using DDR gene mutations in conjunction with clinical criteria to select patients most likely to benefit from cisplatin-based chemotherapy who might be managed with an organ-sparing approach (AO31701 and RETAIN)
Presented by: Gopa Iyer, MD, Memorial Sloan Kettering Cancer Center, New York, NY
Co-Authors: Catherine Tangen, Michal Sarfaty, Ashley Marie Regazzi, I-Ling C. Lee, Woonyoung Choi, Colin P.N. Dinney, Thomas W. Flaig, Ian M Thompson, David James McConkey, Jonathan E. Rosenberg
Affiliations: Fred Hutchinson Cancer Research Center, Seattle, WA, Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel, The University of Texas MD Anderson Cancer Center, Houston, TX, Johns Hopkins Greenberg Bladder Cancer Institute, Department of Urology, Johns Hopkins, Baltimore, MD, University of Colorado Cancer Center, Aurora, CO, Children's Hospital of San Antonio, San Antonio, TX, Johns Hopkins Greenberg Bladder Cancer Institute, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


