(UroToday.com) BCG therapy in non-muscle invasive bladder cancer induces cytokine release that activates NK and T immune cells and results in bladder cancer cell killing and immune memory against further tumor growth. It has been hypothesized that the IL-15 superagonist N-803 could boost the immune response to BCG, thus augmenting patient responses to BCG therapy and limit the need for cystectomy in this disease context.

To validate this hypothesis in patients, Dr. Chamie presented results from the QUILT 3032 phase 2/3 study using the IL-15 superagonist N-803 in combination with BCG in previously BCG-unresponsive bladder cancer. The schema of the study is shown below, with a primary endpoint of complete response. There were two patient cohorts: Cohort A with carcinoma in situ +/- papillary tumors and Cohort B with papillary tumors only.

The characteristics of patients enrolled on this study are shown below, notable for heavy pre-treatment with BCG and other therapies.

Toxicities seen are illustrated below, with rare systemic side effects as would be expected from local intravesical therapy.

In Cohort A (CIS +/- papillary tumors), the complete response rate to BCG + N=803 was 71%, with a median duration of response of over two years. Only 16% of patients went on to cystectomy and 100% of patients were alive despite their bladder cancer diagnosis at the data cut-off.


There was no clear difference in clinical benefit when stratified by various subgroups, as shown below.

Dr. Chamie then compared their results from cohort A to those from KEYNOTE-057, with the caveat that these are not head-to-head randomized comparisons. Overall, response rates and duration of response was higher, with similar and minimal toxicity with both treatment regimens.

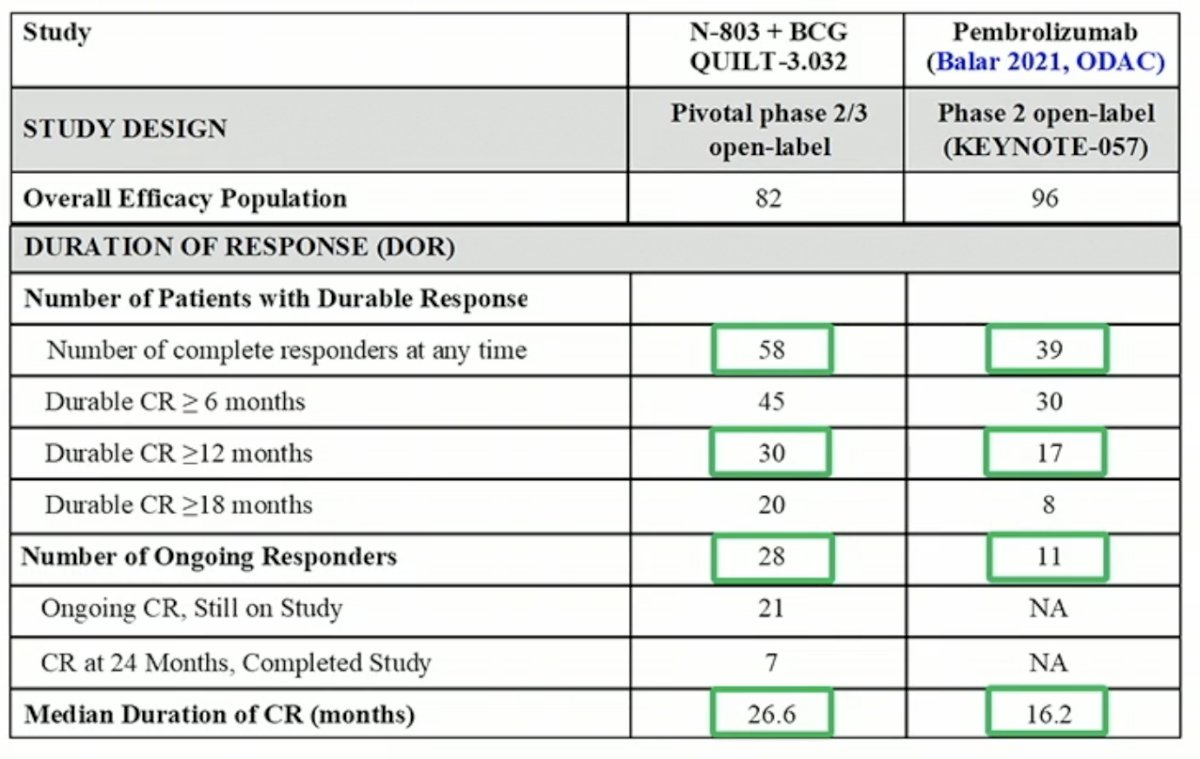
Dr. Chamie then shared results from cohort B of this study. Efficacy was similar across all tested subgroups (age, gender, race, T-stage, ECOG status).

For concisce summary, Dr. Chamie shared the slides below:
Cohort A (CIS +/- papillary tumors)

Cohort B (Papillary tumors)

Dr. Chamie concluded that N-803 added clinically meaningful benefit to BCG in BCG-unresponsive NMIBC.
Presented by: Karim Chamie, MD, Department of Urology, University of California-Los Angeles
Written by: Alok K. Tewari, MD, PhD, medical oncologist at the Dana-Farber Cancer Institute, @aloktewar on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


