(UroToday.com) Cabozantinib is a mult-targeting tyrosine kinase inhibitor of the VEGFR, MET, and AXL proteins that has shown activity in progressive urothelial cancer previously treated with platinum-based chemotherapy. The ATLANTIS trial is an adaptive platform, biomarker stratified, multi-arm study of maintenance therapy in patients with metastatic urothelial carcinoma who experience at least stable disease or better in response to first line chemotherapy.
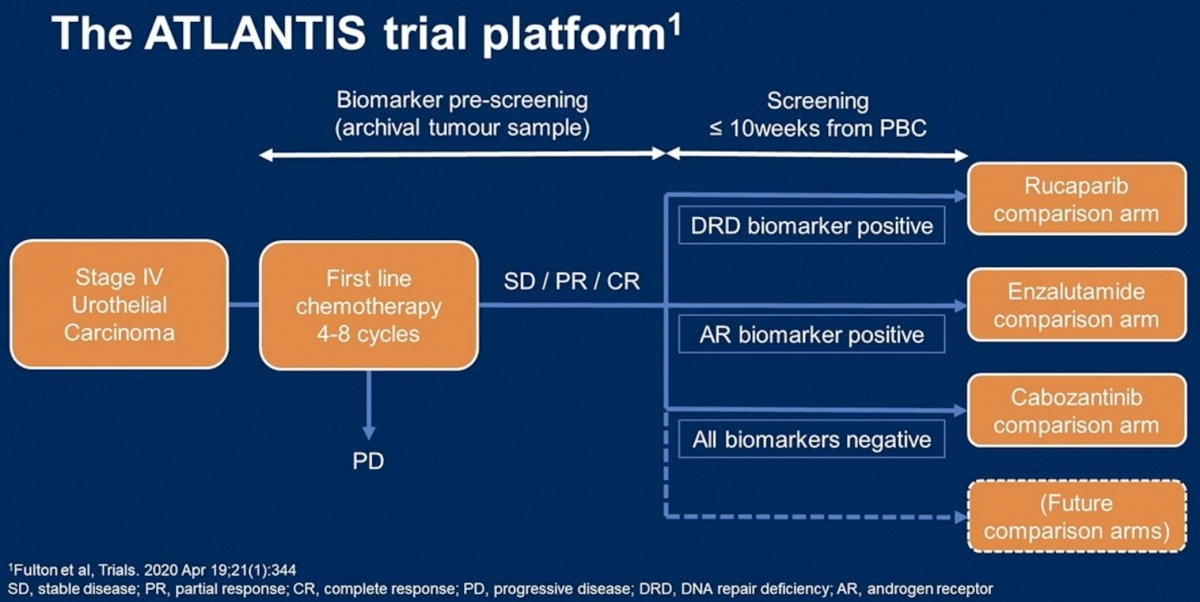
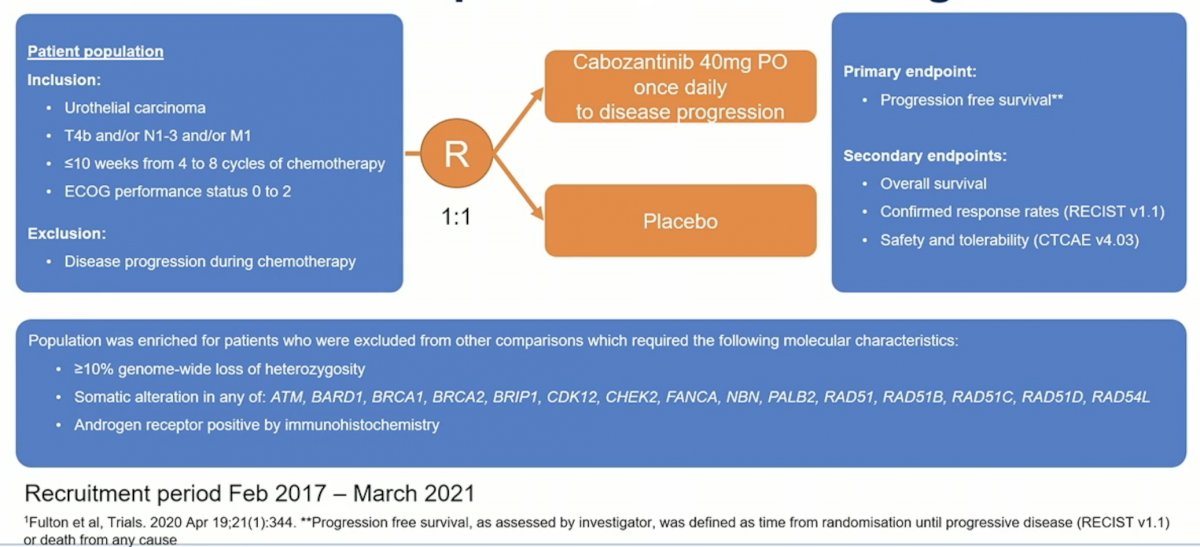
In this presentation, Dr. Jones presented data from the cabozantinib versus placebo biomarker-negative arm of ATLANTIS. The schema and inclusion criteria are indicated below. The primary endpoint of this study was progression free survival.
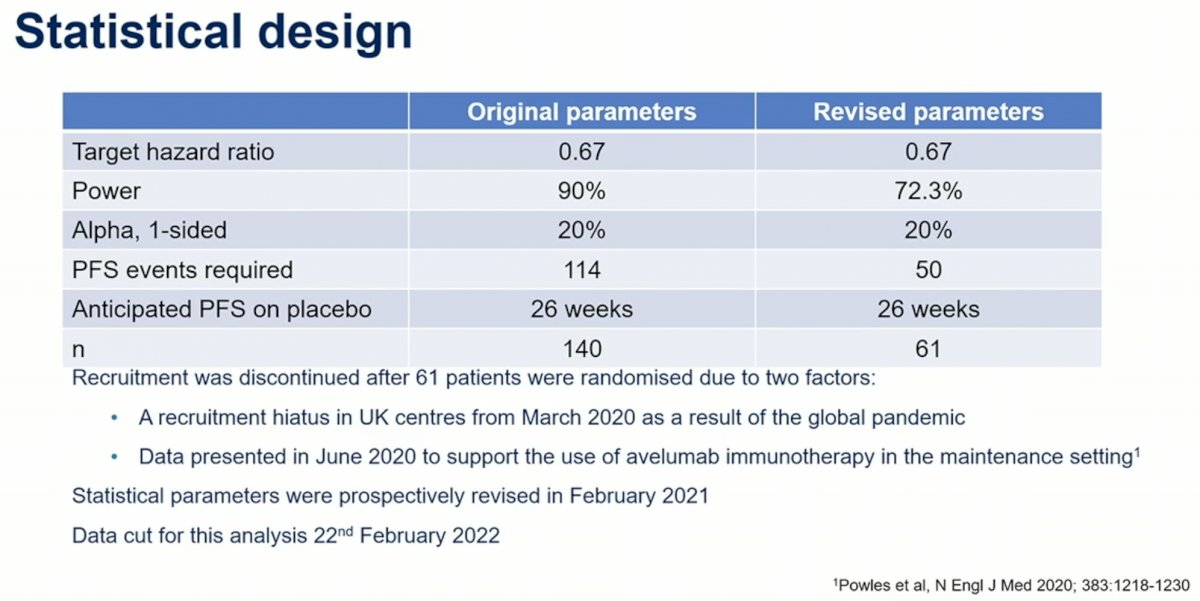
This was a randomized phase 2 screening study, with a relatively high 1-sided alpha parameter built in of 20%, which would allow for a higher rate of false positives for the primary endpoint of PFS. Initially 140 patients were planned, but recruitment was discontinued after 61 patients due both to the covid-19 pandemic as well as data from JAVELIN Bladder 1001 suggesting that avelumab maintenance be a standard of care therapy for these patients. This reduced the overall power of the study.
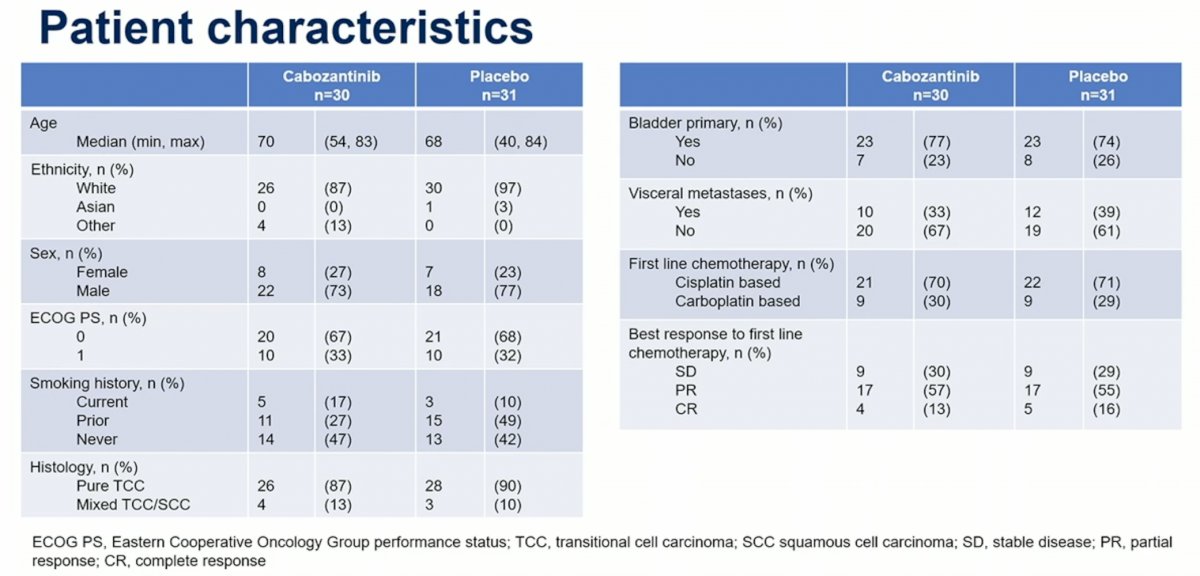
A total of 70% of patients enrolled in this non-biomarker driven arm received first line cisplatin chemotherapy, with the rest receiving carboplatin.
With regards to the primary endpoint of investigator-assessed progression free survival, the median PFS difference between the cabozantinib maintenance and placebo arms was not stoicallycally significant.
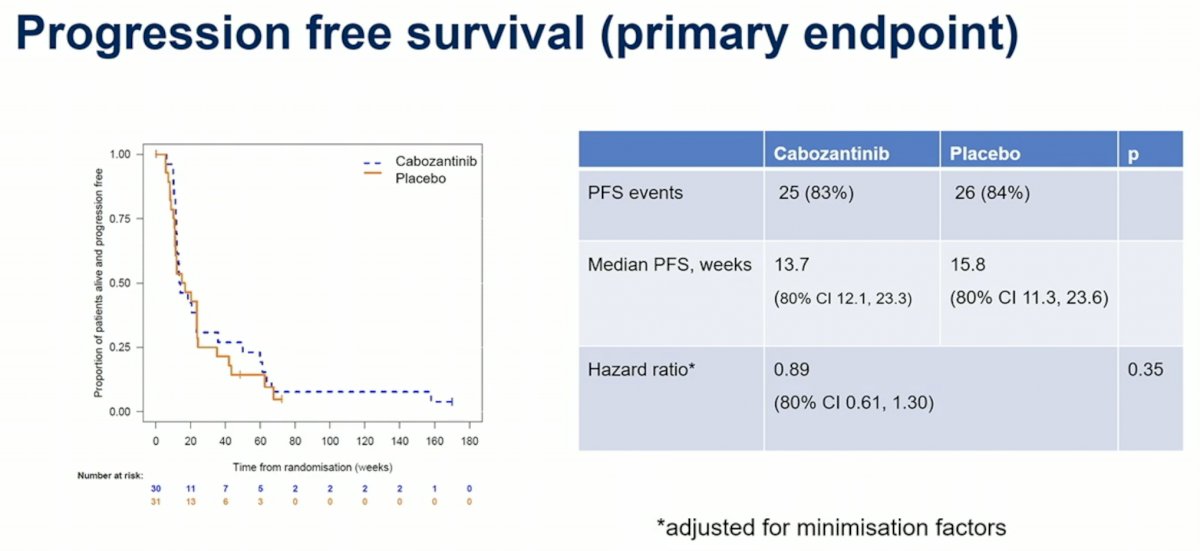
The median overall survival was 75.5 weeks in the cabozantinib arm and 82.9 weeks in the placebo arm, which was not a statistically significant difference.
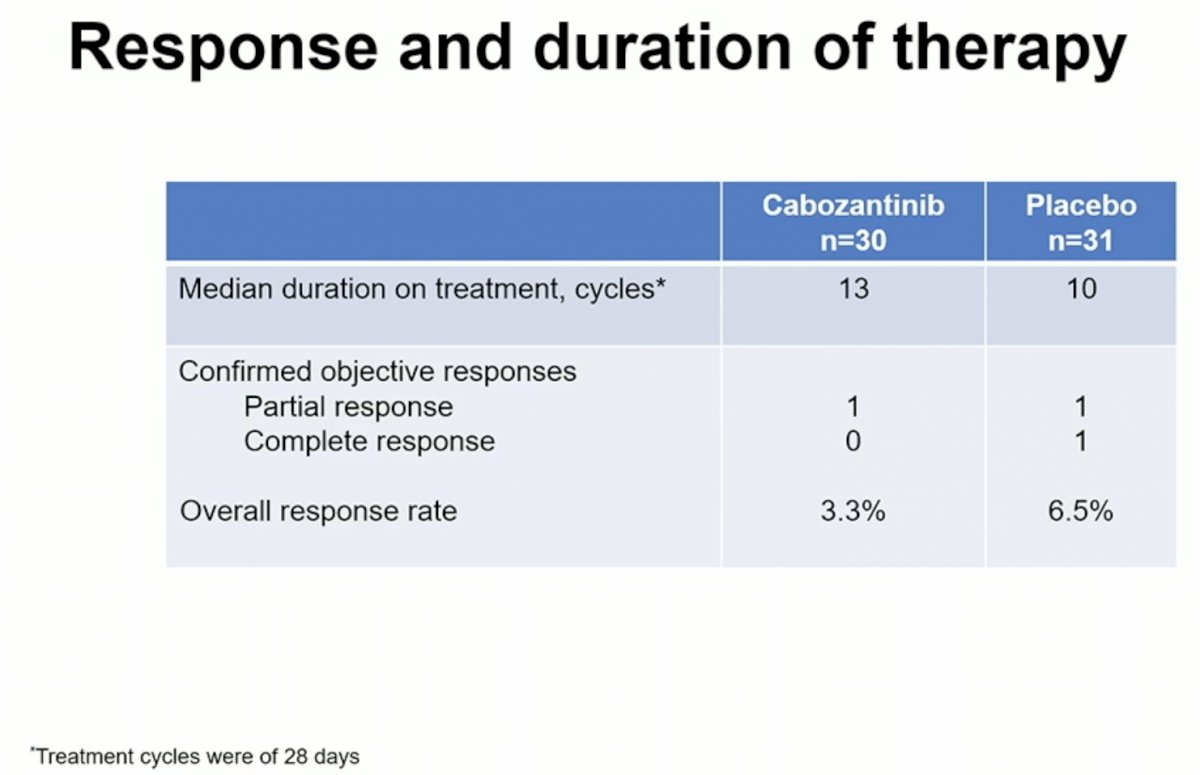
Overall, 43% of patients required a dose reduction in their cabozantinib treatment due to known side effects of the drug.
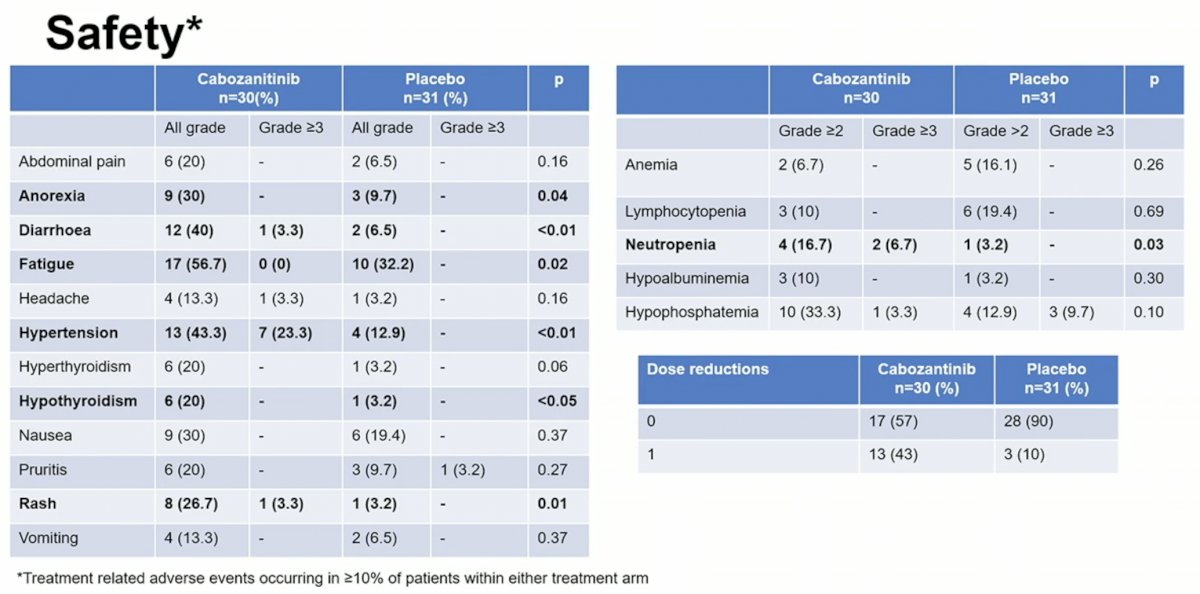
Dr. Jones concluded that this study does not support further investigation of cabozantinib maintenance therapy after at least stable disease in response to first line chemotherapy in advanced urothelial cancer. Based on the JAVELIN Bladder 100 data, placebo is no longer an acceptable control arm for future trials in this disease context, and such efforts should consider combining novel agents with maintenance immunotherapy.
Presented by: Robert J. Jones, MA, PhD, MBChB University of Glasgow, Glasgow, United Kingdom
Written by: Alok K. Tewari, MD, PhD, medical oncologist at the Dana-Farber Cancer Institute, @aloktewar on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.
References:


