(UroToday.com) In this discussion, Dr. Gupta reviewed data presented in abstracts 4505 and 4506 that evaluated cabozantinib as either combination therapy with atezolizumab or single-agent maintenance therapy in advanced urothelial cancer. She started by reviewing the treatment landscape for this disease. If medically eligible, platinum-based chemotherapy remains the unbeaten first-line treatment choice, though data emerging from the EV-302 study may alter this paradigm somewhat. Beyond FGFR inhibitor therapy with erdafitinib, other targeted therapy options are lacking for this disease.

The rationale for targeting the VEGF pathway in urothelial carcinoma is well-established. It stimulates mitogenic activity and angiogenesis, promotes disease progression, and VEGF levels in serum and tissue correlate with disease aggressiveness.

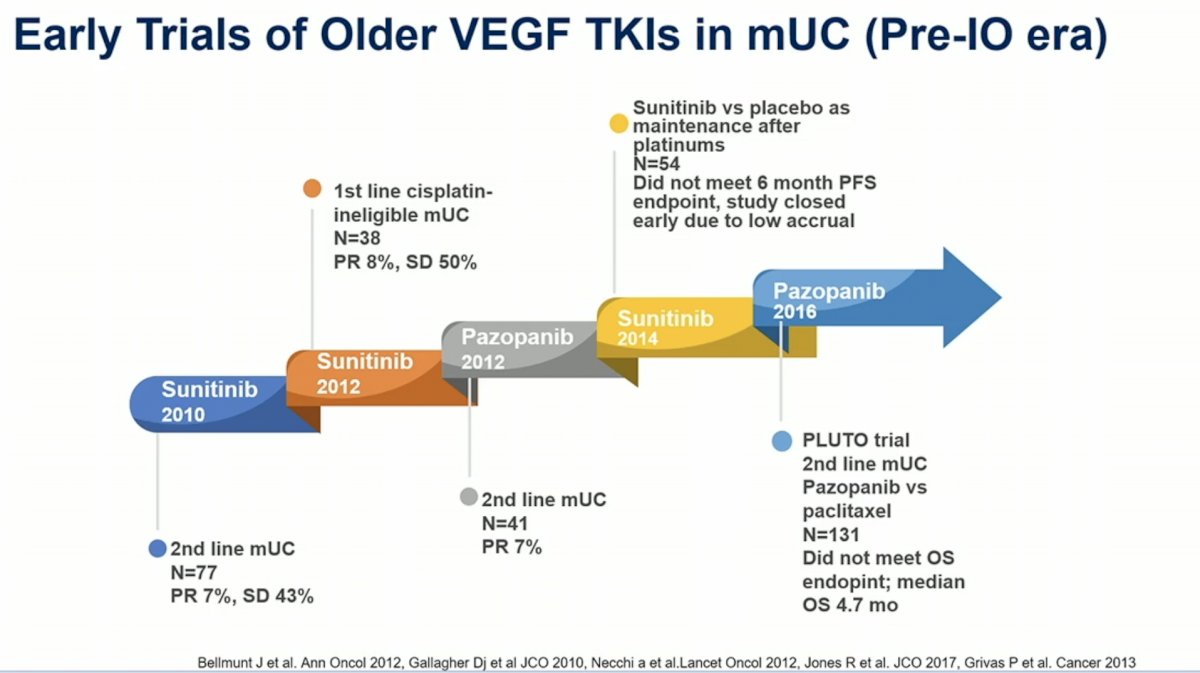
Prior studies of VEGF tyrosine kinase inhibitors such as sunitinib and pazopanib in the second-line or first line platinum-ineligible advanced urothelial carcinoma setting were notable for low response rates, low accrual and toxicity. Cabozantinib is a newer tyrosine kinase inhibitor that targets VEGFR, MET, AXL and RET, and has higher activity than other TKIs in advanced urothelial carcinoma1.
Dr. Gupta first discussed data from the ATLANTIS platform, testing the efficacy of cabozantinib maintenance therapy in patients with metastatic urothelial carcinoma who had at least stable disease on initial platinum-based chemotherapy. She first noted that of the patients screened for this trial, 54 patients were stratified into biomarker driven groups (androgen receptor expression, DNA damage repair alterations), and so there may have been bias against clinical activity in the biomarker-negative group. Second, the power of the study was limited due to a halt in accrual due to the pandemic as well as the establishment of maintenance avelumab as standard of care in this disease context. With these caveats, Dr. Gupta was most impressed by the median duration of treatment (13 28-day cycles on cabozantinib versus 10 28-day cycles of placebo), which is a metric of some significance in the maintenance setting. However, overall she agreed with ATLANTIS investigators that the presented data did not justify ongoing investigation of cabozantinib monotherapy maintenance due to a lack of progression-free survival advantage relative to placebo as well as the new standard of care of maintenance immunotherapy in this setting. This is in contrast with data presented at ASCO GU 2022 from the DNA-damage repair (DDR) alteration arm of ATLANTIS, which suggested a statistically significant PFS benefit for maintenance rucaparib relative to placebo in DDR biomarker-positive patients. Biomarker studies may help further understand which patients could benefit from cabozantinib maintenance therapy in future studies using this agent in combination with other treatments such as immunotherapy.
Dr. Gupta then discussed abstract 4505, which presented results from cohorts 3, 4, and 5 of the COSMIC-021 study combining cabozantinib with atezolizumab in advanced urothelial cancer. Cabozantinib may be immunomodulatory by inhibiting the immunosuppressive effects of VEGF.

The study design is shown below, with patients in cohorts 3 and 4 not having received prior systemic therapy, whereas cohort 5 had received prior immunotherapy.

Dr. Gupta was most encouraged by the disease control rates (80% in cohort 3 and 63% in cohort 4). With the caveat of small numbers, the duration of response does seem to be lower than that reported in IMVigor 210 with single agent atezolizumab. The overall response rate in the prior immunotherapy cohort 5 seemed to be similar to that reported by other investigators with combination cabozantinib and nivolumab (ORR 16% versus 10% in this study, overall small patients cohort size). Overall, she concluded that cabozantinib does not seem to be adding much benefit to immunotherapy in these cohorts, but that combination cabozantinib plus immune checkpoint inhibitor therapy is a compelling maintenance therapy option after platinum chemotherapy. This is being evaluated in the phase 3 MAINCAV study, with the schema for that study shown below.

Presented by: Shilpa Gupta, MD, Taussig Cancer Institute, Cleveland Clinic Foundation
Written by: Alok K. Tewari, MD, PhD, medical oncologist at the Dana-Farber Cancer Institute, @aloktewar on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.
References:
1. Andrea Apolo, Rosa Nadal, Yusuke Tomita, et al. “Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: an open-label, single-centre phase 2 trial.” Lancet Oncol;21(8):1099-1109.


