(UroToday.com) At the 2022 American Society of Clinical Oncology Annual Meeting held in Chicago and virtually, the poster discussion session focused on Kidney and Bladder cancers on Saturday evening included a presentation from Dr. Xinan Sheng on the role of combined treatment of advanced urothelial carcinoma (UC) using a combination of a HER-2 targeted antibody-drug conjugate and immunotherapy.
For patients with metastatic urothelial carcinoma (UC), platinum-based chemotherapy remains the first-line treatment of choice for patients who are eligible. Despite being the guideline-recommended approach, most patients will have disease progression following platinum-based chemotherapy. Immunotherapy using single agent immune checkpoint inhibitors is a standard, guideline-recommended second line treatment. However, the anti-HER-2 antibody-drug conjugate RC48-ADC (Disitamab Vedotin) has shown promising data in HER2-positive patients with locally advanced or metastatic UC who have progression following treatment with platinum-based chemotherapy in RC48-C005 and RC48-C009 trials.
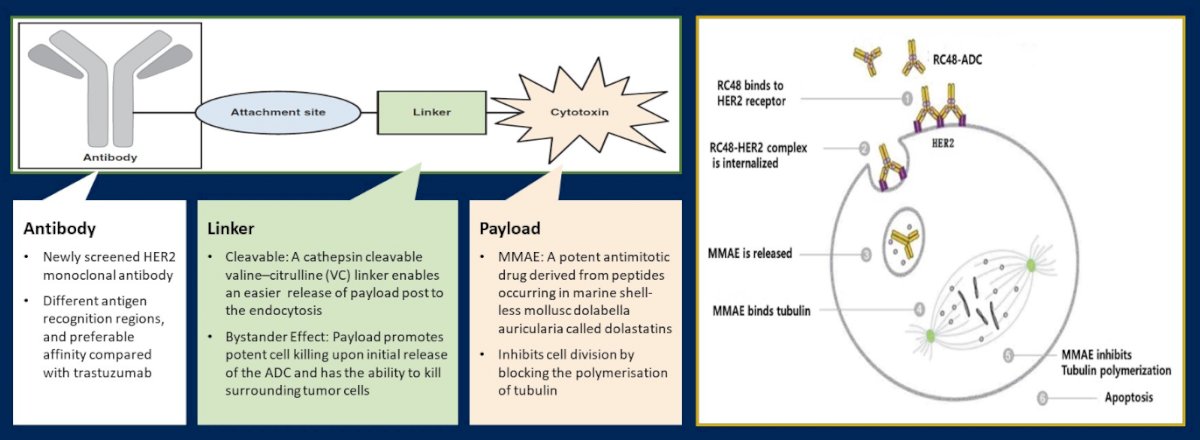
In this abstract, the authors used pooled analysis of these two studies to provide updated efficacy, safety and overall survival data.
Each of the included studies is a single-arm, multi-center, phase II trials. Both trial enrolled patients who were 18-80 years old, with central-laboratory confirmed, histologically HER2-positive (IHC2+,3+), unresectable metastatic UC. All patients had received at least one line of systemic chemotherapy. The primary endpoint was objective response rate (ORR) with secondary outcomes including progression-free survival (PFS), overall survival (OS), and safety endpoints.
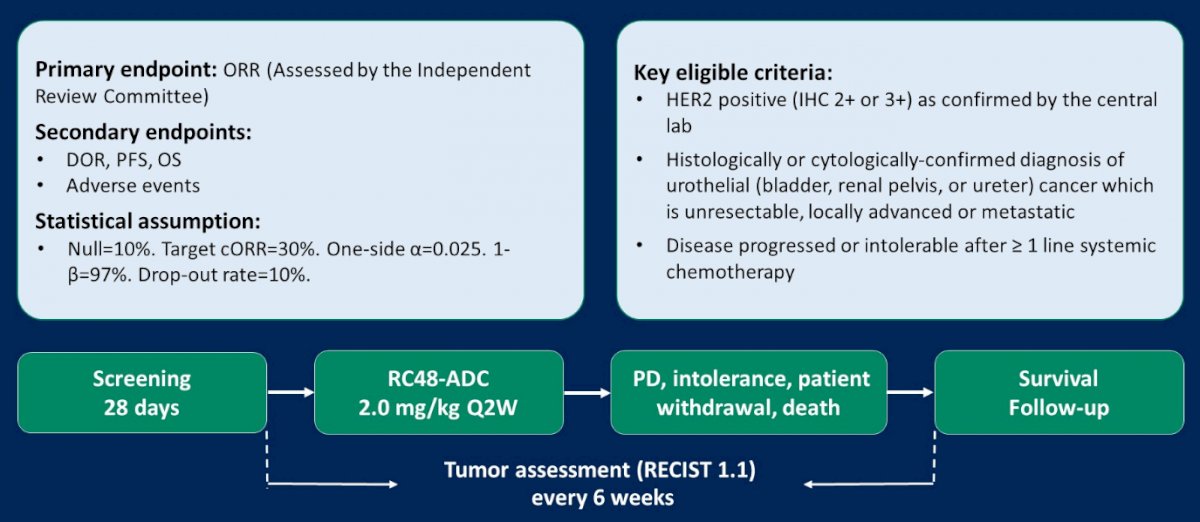
These two trials [RC48-C005 (NCT03507166) and RC48-C009 (NCT03809013)] enrolled patients with HER2-positive locally advanced or metastatic UC from November 2017 to September 2020, including in total 107 patients. The median age of the combined cohort was 63 years (40-79) and 80 of 107 were men. Most (64.5%) patients had received at least two lines of 2 lines systemic chemotherapy and the vast majority (90.7%) had visceral metastases.
With a data cutoff of September 4, 2021, the overall confirmed ORR as assessed by the BIRC was 50.5% (95% CI: 40.6%, 60.3%). The disease control rate (DCR) was 82.2% (95% CI:73.7%, 89.0%).
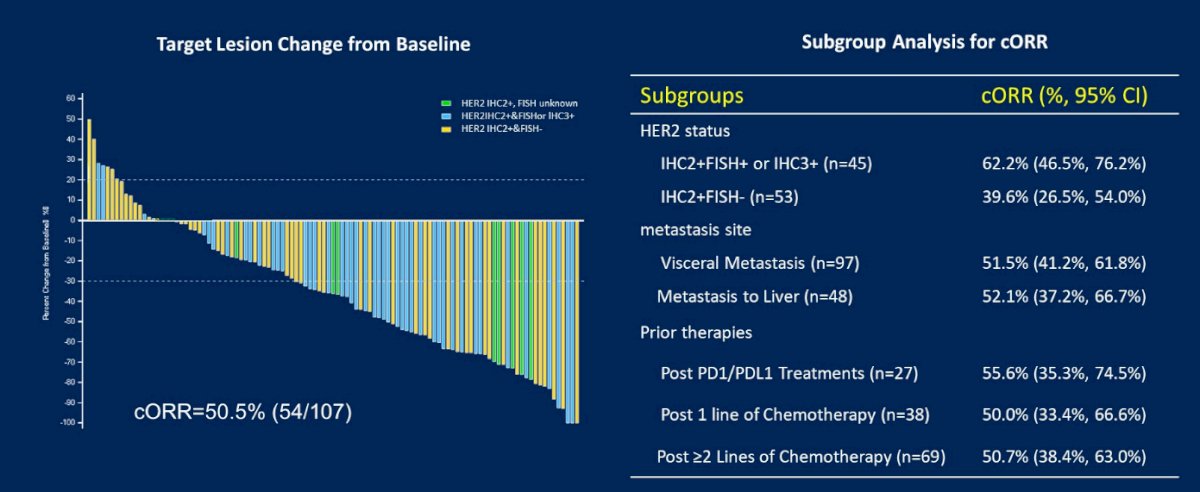
The cORR was generally consistent across prespecified subgroups. The cORR was 52.1% (25/48) for patients with liver metastasis and was 55.6% (15/27) in patients with prior PD-1/L1 treatment. The cORR was 62.2% (28/45) for HER2 IHC2+&FISH+ or IHC3+ patients, 55.6% (5/9) for HER2 IHC2+&FISH unknown patients, and 39.6% (21/53) for HER2 IHC2+&FISH- patients respectively.
The median PFS and OS were 5.9 (95% CI:4.2, 7.2) months and 14.2 (95% CI:9.7, 18.8) months, respectively, over a median follow-up of 19.1 months.
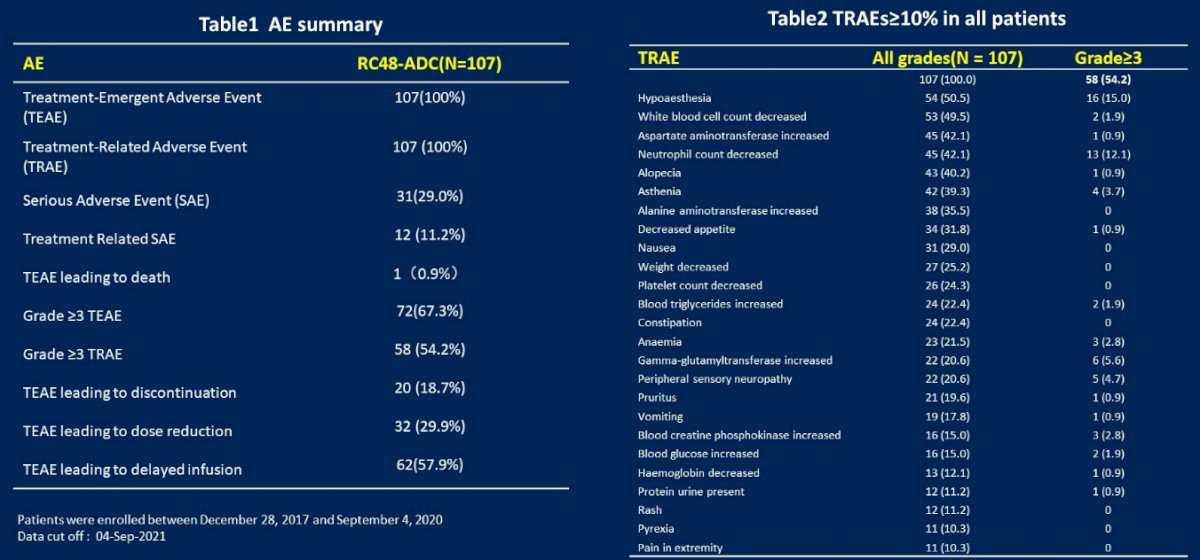
The most common treatment-related AEs were hypoaesthesia (50.5%), leukopenia (49.5%), aspartate aminotransferase (AST) increased (43.0%), neutropenia (42.1%), alopecia (40.2%), asthenia (39.3%), alanine aminotransferase (ALT) increase (35.5%), and decreased appetite (31.8%). In terms of serious adverse event, common grade ≥3 TRAEs (≥5%) only included hypoaesthesia (15.0%), neutropenia (12.1%) and r-GT increased (5.6%).
Thus, the authors conclude that RC48-ADC monotherapy showed a promising efficacy with a manageable safety profile in HER2-positive patients with metastatic UC who progressed following at least one line of systemic chemotherapy.
Presented by: Xinan Sheng, MD, ABFT, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Genitourinary Oncology, Beijing Cancer Hospital, Beijing, China


