(UroToday.com) At the 2022 American Society of Clinical Oncology Annual Meeting held in Chicago and virtually, the poster discussion session focused on Kidney and Bladder cancers on Saturday evening included a presentation from Dr. Jonathan E. Rosenberg examining long-term outcomes of enfortumab vedotin (EV) in patients with previously treated advanced urothelial carcinoma (UC) in the EV-301 trial.
Following earlier, non-randomized data, the pre-specified interim analysis of the EV-301 trial demonstrated improvements in progression-free and overall survival for patients with previously treated advanced urothelial carcinoma (UC) who received EV compared to chemotherapy. EV is an antibody-drug conjugate directed against Nectin-4, providing a novel mechanism to targeted UC. However, longer term outcomes for EV treatment are unknown and therefore, in this presentation, the authors provide updated results from the EV-301 trial with 12 additional months of follow-up.
While previously presented and published, in brief, EV-301 is a confirmatory phase 3, randomized, open-label randomized controlled trial (NCT03474107) that enrolled patients with locally advanced or metastatic urothelial carcinoma who had received a prior platinum-containing chemotherapy and had disease progression during or after PD-1/L1 inhibitor treatment. Enrolled patients were randomized in a 1:1 fashion to receive either EV 1.25 mg/kg on Days 1, 8, and 15 of each 28-day cycle or investigator-chosen standard chemotherapy with docetaxel, paclitaxel, or vinflunine.

The primary endpoint was OS with a number of secondary endpoints including investigator-assessed PFS per RECIST v1.1, as well as safety and tolerability. While the interim analysis was based off a data cutoff of July 15, 2020, this abstract provides updated efficacy and safety findings with a data cutoff of July 30, 2021.
The authors randomized 608 patients with locally advanced or metastatic UC to EV (n = 301) or chemotherapy (n = 307). As of the data cutoff of July 30, 2021, 444 deaths had occurred (EV, n = 207; chemotherapy, n = 237).
With a 23.75-month median follow-up, the median OS was significantly longer among patients receiving EV (12.91 months) compared to chemotherapy (8.94 months), a difference of 3.97 months (HR = 0.704 [95% CI: 0.581-0.852], 1-sided P= 0.00015).
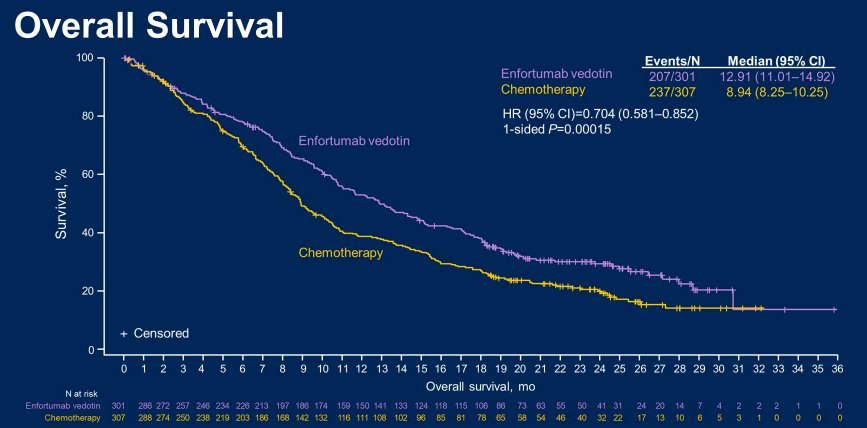
Additionally, the OS benefit of EV was retained in the majority of prespecified subgroups.
Examining secondary outcomes, PFS also was significantly improved with EV (median 5.55 months) vs chemotherapy (median 3.71 months) (HR = 0.632 [95% CI: 0.525-0.762]; 1-sided P< 0.00001). Further, rates of treatment-related adverse events (TRAEs; 93.9% vs 91.8%), including serious TRAEs (22.6% vs 23.4%), were comparable between the treatment arms. High grade (grade ≥3) TRAEs occurred in 50% of patients in both groups.

Thus, the authors conclude that EV continues to show a significant and consistent survival advantage over standard chemotherapy in patients with previously treated locally advanced and metastatic UC, without evidence of new safety signals over longer follow-up.
Presented by: Jonathan E. Rosenberg, MD, Chief of the Genitourinary Medical Oncology Service, Division of Solid Tumor Oncology; and the Enno W. Ercklentz Chair at Memorial Sloan Kettering Cancer Center, New York City, New York


