(UroToday.com) The 2022 ASCO annual meeting featured a poster discussion session on kidney and bladder cancer, including a presentation by Dr. Tom Powles discussing an analysis of progression after first subsequent therapy in KEYNOTE-426, which tested pembrolizumab + axitinib versus sunitinib as first-line therapy for advanced clear cell RCC. The randomized, open-label, phase 3 KEYNOTE-426 study (NCT02853331)1 met its primary and key secondary end points of improved OS, PFS, and ORR with pembrolizumab + axitinib versus sunitinib as first-line treatment for patients with advanced clear cell RCC. Extended follow-up (42.8-month median follow-up) continued to show the superior efficacy of pembrolizumab + axitinib versus sunitinib in this patient population.2 This exploratory analysis compared the benefit of pembrolizumab + axitinib versus sunitinib beyond initial progression by evaluating PFS2 for all randomly assigned patients and across IMDC risk categories.
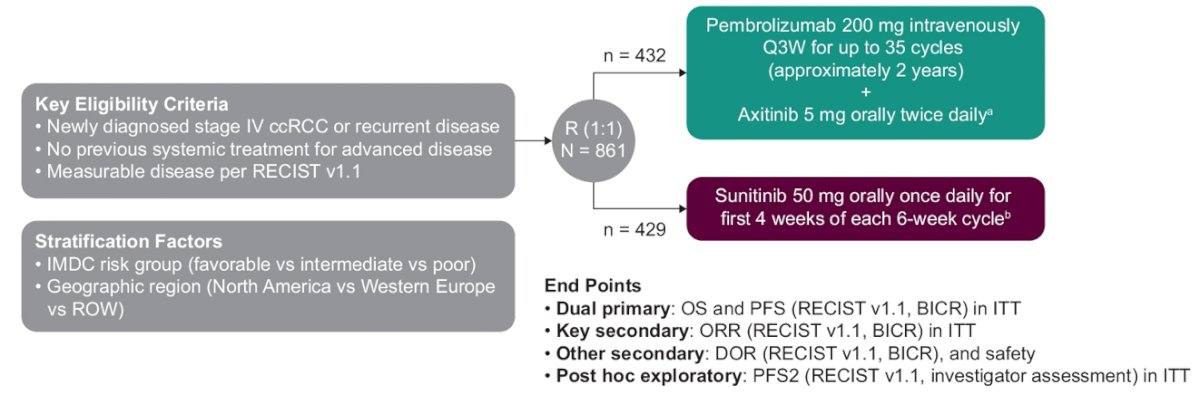
Treatment-naive patients with advanced clear cell RCC, Karnofsky Performance Status Scale score ≥70% and measurable disease per RECIST v1.1 were randomly assigned 1:1 to receive pembrolizumab 200 mg IV every 3 weeks for up to 35 doses (̃2 years) + axitinib 5 mg orally twice daily or sunitinib 50 mg orally once daily on a 4-week on/2-week off schedule. The study design for KEYNOTE-426 is as follows:
The end point of this exploratory analysis was PFS2, defined as time from randomization to progression after first subsequent therapy or any-cause death. The Kaplan-Meier method was used to estimate PFS2 and hazard ratios were estimated using a Cox regression model.
Of 861 patients, 432 were assigned to receive pembrolizumab + axitinib and 429 to sunitinib. Median time from randomization to the database cutoff date (January 11, 2021) was 42.8 months (range, 35.6-50.6). Overall, 47.2% of patients (204/432) in the pembrolizumab + axitinib arm and 65.5% of patients (281/429) in the sunitinib arm received ≥1 line of subsequent anticancer therapy. For patients who received subsequent therapy, anti–PD-1/PD-L1 agents were the first subsequent treatment for 11.3% of patients (23/204) in the pembrolizumab + axitinib arm and 54.8% of patients (154/281) in the sunitinib arm. In the pembrolizumab + axitinib arm, 82.8% of patients (169/204) received a VEGF/VEGFR inhibitor as first subsequent therapy, as did 43.4% (122/281) in the sunitinib arm. The following figure summarizes first-subsequent anticancer therapy:
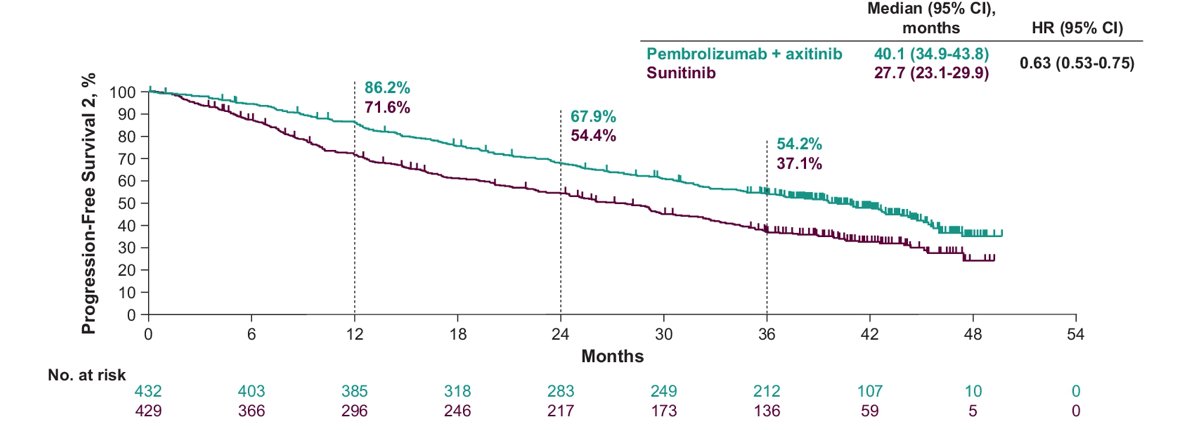
In the ITT population, the median time to PFS2 was 40.1 months for pembrolizumab + axitinib versus 27.7 months for sunitinib (HR 0.63, 95% CI 0.53-0.75):
Among patients with IMDC favorable risk disease, the median time to PFS2 was 46.0 months for pembrolizumab + axitinib versus 39.9 months for sunitinib (HR 0.68, 95% CI 0.47-0.98):

Among patients with IMDC intermediate/poor risk disease, the median time to PFS2 was 32.1 months for pembrolizumab + axitinib versus 20.1 months for sunitinib (HR 0.62, 95% CI 0.51-0.76):

Dr. Powles concluded his presentation discussing analysis of progression after first subsequent therapy in KEYNOTE-426 with the following take-home messages:
- Long-term results of KEYNOTE-462 continue to support pembrolizumab + axitinib as standard of care for patients with previously untreated advanced clear cell RCC
- PFS2 was longer for patients in the pembrolizumab + axitinib group than for those in the sunitinib group, regardless of IMDC risk group
- Results from this exploratory analysis of PFS2 support the long-term benefit of pembrolizumab + axitinib as first-line treatment for patients with advanced clear cell RCC
Presented by: Thomas Powles, MD, Barts Health NHS Trust and the Royal Free NHS Foundation Trust, Barts Cancer Institute, and Queen Mary University of London, London, United Kingdom
Co-Authors: Elizabeth R. Plimack, Viktor Stus, Tom Waddell, Rustem Gafanov, Frédéric Pouliot, Dmitry Nosov, Bohuslav Melichar, Denis Soulieres, Delphine Borchiellini, Ihor Vynnychenko, Raymond S. McDermott, Sergio Jobim Azevedo, Satoshi Tamada, Anna Kryzhanivska, Chenxiang Li, Joseph E. Burgents, L. Rhoda Molife, Brian I. Rini, Jens Bedke
Affiliations: Fox Chase Cancer Center, Philadelphia, PA, Dnipro State Medical University, Dnipro, Ukraine, The Christie NHS Foundation Trust, Manchester, United Kingdom, Russian Scientific Center of Roentgenoradiology, Moscow, Russian Federation, CHU of Québec and Laval University, Québec City, ON, Canada, Central Clinical Hospital With Outpatient Clinic, Moscow, Russian Federation, Palacky University Medical School and Teaching Hospital, Olomouc, Czech Republic, Centre Hospitalier de l’Universitaire de Montréal, Montréal, QC, Canada, Centre Antoine Lacassagne, Université Côte d’Azur, Nice, France, Sumy State University, Sumy Regional Oncology Center, Sumy, Ukraine, Adelaide and Meath Hospital and University College Dublin, Dublin, Ireland, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil, Bell Land General Hospital, Osaka, Japan, Ivanko-Frankivsk National Medical University, Ivano-Frankivsk, Ukraine, Merck & Co., Inc., Kenilworth, NJ, MSD UK, London, United Kingdom, Vanderbilt-Ingram Cancer Center, Nashville, TN, Eberhard Karls Universität Tübingen, Tübingen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.
References:
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1116-1127.
- Powles T, Plimack ER, Soulieres D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomized, open-label, phase 3 trial. Lancet Oncol. 2020 Dec;21(12):1563-1573.



