(UroToday.com) At the 2022 American Society of Clinical Oncology Annual Meeting held in Chicago and virtually, the oral abstract session focused on Prostate, Testicular, and Penile cancers on Sunday morning included a presentation from Dr. Michael Hofman providing updated results of the TheraP trial, a randomized comparison of 177Lu-PSMA-617 (LuPSMA) and cabazitaxel in men with metastatic castration-resistant prostate cancer (mCRPC) following docetaxel chemotherapy.
Theranostic treatment using LuPSMA has rapidly gained interest in both research and clinical practice spheres. The principle of theranostic approaches rely on targeted delivery of treatment directly to cancer cells: in the case of 177Lu-PSMA-617 (LuPSMA), the beta-emitter lutetium is targeted to prostate cancer cells using antibodies to the transmembrane protein PSMA (prostate specific membrane antigen).

TheraP (NCT03392428) was the first randomized trial of this treatment approach showing that, in men with mCRPC who had disease progression after docetaxel, those randomly assigned to LuPSMA has significant improvements in PSA response rate (66% vs. 37%), RECIST response rate (49% vs. 24%), progression-free survival (HR 0.63), less grade 3 or 4 toxicities (33% vs. 53%) and better patient-reported outcomes, compared to those who received cabazitaxel.
At this year’s ASCO meeting, Dr. Hofamn provided results regarding the secondary endpoint of overall survival (OS) with mature follow-up, as well as data on those patients initially excluded because of low PSMA-expression or discordant disease on imaging with PSMA-PET and FDG-PET.
The design of the TheraP trial has previously been described and published. In short, the authors accrued men with mCRPC progressing after docetaxel who had PET imaging with 68Ga-PSMA-11 that showed high PSMA-expression (at least one site with SUVmax≥20) and 18F-FDG demonstrating no sites of disease of FDG-positive and PSMA-negative (discordant disease). Once enrolled, participants were randomly assigned in a 1:1 fashion to LuPSMA (8.5-6GBq every 6 weeks, maximum 6 cycles) or cabazitaxel (20mg/m2 every 3 weeks, maximum 10 cycles).

The key secondary endpoint of overall survival was analyzed by intention-to-treat and summarized by restricted mean survival time (RMST) to account for non-proportional hazards.
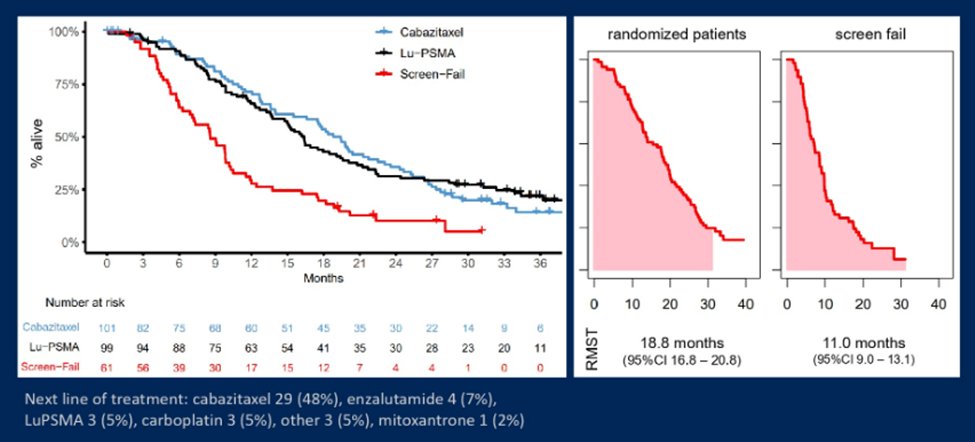
Between February 6, 2018 and September 3, 2019, 291 patients were screened and 200 were randomly assigned, either to (99) or cabazitaxel (101). Following initial eligibility assessment, 80 of 291 (27%) men were excluded after PSMA/FDG-PET: 51 had an SUVmax that was less than 20 and 29 had discordant lesions between PSMA/FDG-PET. Follow-up is available for 61 of these 80 men (76%).
In a brief reassessment of progression-free survival, Dr. Hofman noted that there was a “pinching of the curve” around 6 months suggesting non-proportional hazards. As a result, the trials statistical team opted to use RMST for analysis of both progression-free survival and overall survival.

After a median follow-up time of 36 months (data cut-off December 31, 2021), 70 patients randomly assigned to cabazitaxel had died (of 101), 77 (of 99) assigned LuPSMA had died, and 55 (of 61) excluded after PSMA/FDG-PET had died. Subsequent, post-protocol treatments were given relatively frequently. Among those assigned cabazitaxel, 21 patients received further cabazitaxel in 21 and 20 received LuPSMA. Among those assigned LuPSMA, 5 patients received additional LuPSMA and 32 received cabazitaxel.
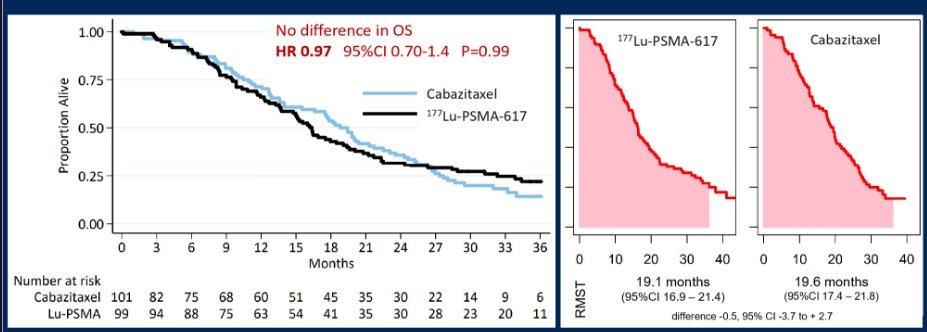
Overall survival was similar in those randomly assigned LuPSMA versus cabazitaxel (RMST to 36 months was 19.1 vs. 19.6 months, difference -0.5, 95% CI -3.7 to + 2.7). Among 61 men excluded by imaging with PSMA/FDG-PET before randomisation, RMST to 36 months was 11.0 months (95% CI 9.0 to 13.1), following treatment that included cabazitaxel in 29 (48%) and LuPSMA in 3 (5%).
Dr. Hofman then moved to a discussion of screen failures in the initial evaluation period of the TheraP trial. These screen failures were due to either low PSMA expression or PSMA/FDA discordance. As noted in the figures below, patients who failed screening had a substantially worse overall survival and lower RMST.
No additional safety signals were identified with longer follow-up. Thus, when we look to apply these data to practice, Dr. Hofman highlighted the strengths of TheraP including the prospective, randomized, multi-center design, three years of follow-up, and use of an active control arm. However, there was substantial post-protocol cross-over as well as post-randomization withdrawal among men in the cabazitaxel arm which confound the assessment of overall survival. However, it is clear that LuPSMA is more active than cabazitaxel (as noted by PSA response rates, radiographic progression-free survival, and PSA-progression-free survival) with similar overall survival to cabazitaxel (a proven life-prolonging therapy) with fewer adverse events and better patient reported outcomes.
Further, Dr. Hofman noted that PSMA uptake may act as a predictive biomarker: in patients with PSMA SUVmean levels of 10 of greater, the odds of response to LuPSMA were substantially higher (OR 12.2, 95% CI 3.4-59) as compared to those with PSMA SUVmean of less than 10 (OR 2.2, 95% CI 1.1-4.5) (p-value for difference = 0.03).
Thus, Dr. Hofman concluded that LuPSMA is a suitable option for men with mCRPC progressing after docetaxel, with lower adverse events, higher response rates, improved patient-reported outcomes, and similar OS compared with cabazitaxel. Median survival was considerably shorter for patients excluded on PSMA/FDG-PET due to either low PSMA expression or FDG-discordant disease who would otherwise be eligible for LuPSMA.
Presented by: Michael S. Hofman, MBBS (Hons), FRACP, FAANMS, Professor of Molecular Imaging, The University of Melbourne, and Nuclear Medicine Physician in the Centre for Cancer Imaging at the Peter MacCallum Cancer Centre in Melbourne, Australia


