(UroToday.com) The 2022 ASCO annual meeting featured an oral abstract session on prostate cancer, including a Prostate Cancer Foundation (PCF.org) and Prostate Cancer UK sponsored presentation by Dr. Susan Halabi discussing intermediate clinical endpoints as potential surrogates for overall survival in men with mHSPC. Overall survival (OS) is the gold standard endpoint in oncology trials, however clinical trials in mHSPC with OS as a primary endpoint take close to a decade to read out. Surrogate endpoints are proposed based on biological pathways, available earlier in the course of the cancer’s natural history, and measured more frequently than the true endpoint (OS). Dr. Halabi and colleagues hypothesized that radiographic progression-free survival (rPFS) and clinical PFS (cPFS) are valid surrogates for OS in men with mHSPC and could potentially be used to expedite phase 3 clinical trials. This hypothesis was investigated by the STOPCAP M1 Collaboration.
Dr. Halabi and colleagues obtained individual patient data from eligible randomized trials comparing treatment regimens (ADT or ADT + docetaxel in the control or research arms) in mHSPC. For this study, the true end point was OS (the date of randomization to date of death from any cause), and intermediate clinical endpoints (rPFS and cPFS) were defined as follows: rPFS was defined as time from randomization to radiographic progression (defined per protocol) or death from any cause, whichever occurred first; cPFS was defined as time from randomization to date of radiographic progression, symptoms, initiation of new treatment, or death, whichever occurred first.

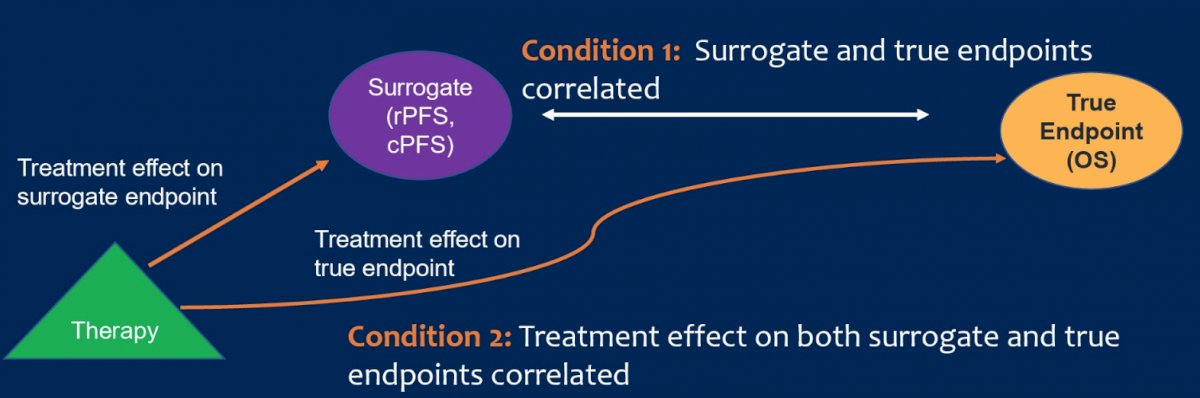
Dr. Halabi implemented a two-stage meta-analytic validation model where conditions of trial level and patient level surrogacy had to be met:
They then computed the surrogate threshold effect, which is the minimum intermediate clinical endpoint treatment effect necessary to estimate a non-zero effect on OS. With regards to data analysis, the following were noted for each condition:
- Condition 1:
- Weighted linear regression Kaplan-Meier estimates of OS at 5 years versus 3 years for intermediate clinical endpoints
- These landmarks were chosen and considered clinically important
- 3-year rPFS data would expedite clinical trial read-out
- 5-year OS is beyond median OS of mHSPC and would be mature
- Proportion of variance (R2) explained by the regressions
- Condition 2:
- Weighted linear regression of log hazard ratio (HR) of OS versus log HR intermediate clinical endpoints
- Proportion of variance (R2)
Individual patient data from 8,592 patients randomized from 1994-2012 from 9 trials (13 comparisons) were pooled for a stratified analysis. The characteristics of the 9 trials is as follows:

There were 5,377 deaths, of which 3,971 (74%) were due to prostate cancer. The median follow-up for surviving patients was 75.6 months. In addition, there were 6,227 rPFS and 6,314 cPFS events. The median OS, rPFS and cPFS were 4.1 years, 2.3 years, and 2.1 years, respectively:

For condition 1, intermediate clinical endpoints and OS were correlated, with an R2 = 0.78 for rPFS and OS (Kendall’s Tau 0.83, 95% CI 0.82-0.84), and R2 = 0.78 for cPFS and OS (Kendall’s Tau 0.84, 95% CI 0.83-0.85). For condition 2, treatment effects on rPFS and OS correlated, with an R2 = 0.81, and for cPFS was R2 = 0.81.
The strengths of the current study were (i) the large number of patients (>8,500 patients) and number of trials (9), which included 5 comparisons from STAMPEDE, (ii) used individual patient level data, allowing an estimate of the association between surrogate and true endpoints, estimate parameters with greater precision, and conduct subgroup analyses, and (iii) intermediate clinical endpoints (rPFS, cPFS) were harmonized across trials and used a protocol definition. One limitation is that disease volume and timing of M1 were not collected prior to the CHAARTED trial.
Dr. Halabi concluded her presentation discussing intermediate clinical endpoints as potential surrogates for overall survival in men with mHSPC with the following take-home messages:
- Both rPFS and cPFS appear to be valid surrogate endpoints for OS in men with mHSPC
- The surrogate threshold effect makes it viable for either rPFS or cPFS to be used as the primary endpoint as a surrogate for OS in phase 3 mHSPC trials and would expedite trial conduct
- Validation of these intermediate clinical endpoints in trials with drugs having other mechanisms of action is planned (testosterone suppression + abiraterone/enzalutamide/apalutamide/darolutamide)
Presented by: Susan Halabi, PhD, Duke University Medical Center, Durham, NC
Co-Authors: Akash Roy, Larysa Rydzewska, Peter Godolphin, Mahesh K. B. Parmar, Maha H. A. Hussain, Catherine Tangen, Ian Thompson, Wanling Xie, Michael Anthony Carducci, Matthew Raymond Smith, Michael J. Morris, Gwenaelle Gravis, David P. Dearnaley, Paul Verhagen, Takayuki Goto, Nicholas D. James, Marc E. Buyse, Jayne F. Tierney, Christopher Sweeney
Affiliations: Duke University, Durham, NC, University College London, London, United Kingdom, MRC Clinical Trials Unit at UCL, London, United Kingdom, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL, Fred Hutchinson Cancer Research Center, Seattle, WA, Christus Health, San Antonio, TX, Dana-Farber Cancer Institute, Boston, MA, Sidney Kimmel Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, Massachusetts General Hospital Cancer Center, Boston, MA, Division of Solid Tumor Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, Institut Paoli-Calmettes Aix-Mareseille Université, Marseille, France, Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London, United Kingdom, Erasmus Medical Center, Rotterdam, Netherlands, Department of Urology, Graduate School of Medicine, Kyoto University, Kyoto, Japan, Institute of Cancer Research, Birmingham, United Kingdom, International Drug Development Institute, Louvain-La-Neuve, Belgium, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


