(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a kidney and bladder cancers poster session. Dr. Sarmad Sadeghi presented the rationale and study design for SWOG S1937, a phase III randomized controlled trial of eribulin, with or without gemcitabine, versus standard of care for metastatic urothelial carcinoma refractory to or ineligible for PD-1 or PD-L1 antibodies.
Dr. Sadeghi noted that urothelial carcinoma remains the 2nd most common genitourinary malignancy. The current first-line, standard of care approach for the management of metastatic urothelial carcinoma remains platinum-based combination chemotherapy with dose-dense methotrexate, vinblastine, adriamycin, and cisplatin (ddMVAC) or gemcitabine-cisplatin (GC). For cisplatin-ineligible patients, current approaches include gemcitabine-carboplatin or pembrolizumab in select patients with high PD-L1 expression. Other options in this disease space include erdafitinib for patients with FGFR alterations and enfortumab vedotin (Nectin-4 directed antibody), which is FDA-approved for patients who have previously received a PD-1/PD-L1 inhibitor and platinum-containing chemotherapy or are cisplatin-ineligible.
Eribulin is a microtubule inhibitor that has previously demonstrated efficacy in patients with metastatic urothelial carcinoma. A phase I/II trial of eribulin in this setting demonstrated an objective response rate (ORR) of 37.5% with a median progression-free survival (PFS) of 4.1 months and median overall survival (OS) of 9.5 months (n=150). A subsequent phase II trial of combination gemcitabine + eribulin in cisplatin-ineligible metastatic urothelial carcinoma patients demonstrated an ORR of 50% with median PFS and OS of 5.3 and 12 months, respectively (n=24). The most common Grade 3-4 toxicities observed were neutropenia 63% and anemia/fatigue 29%.1
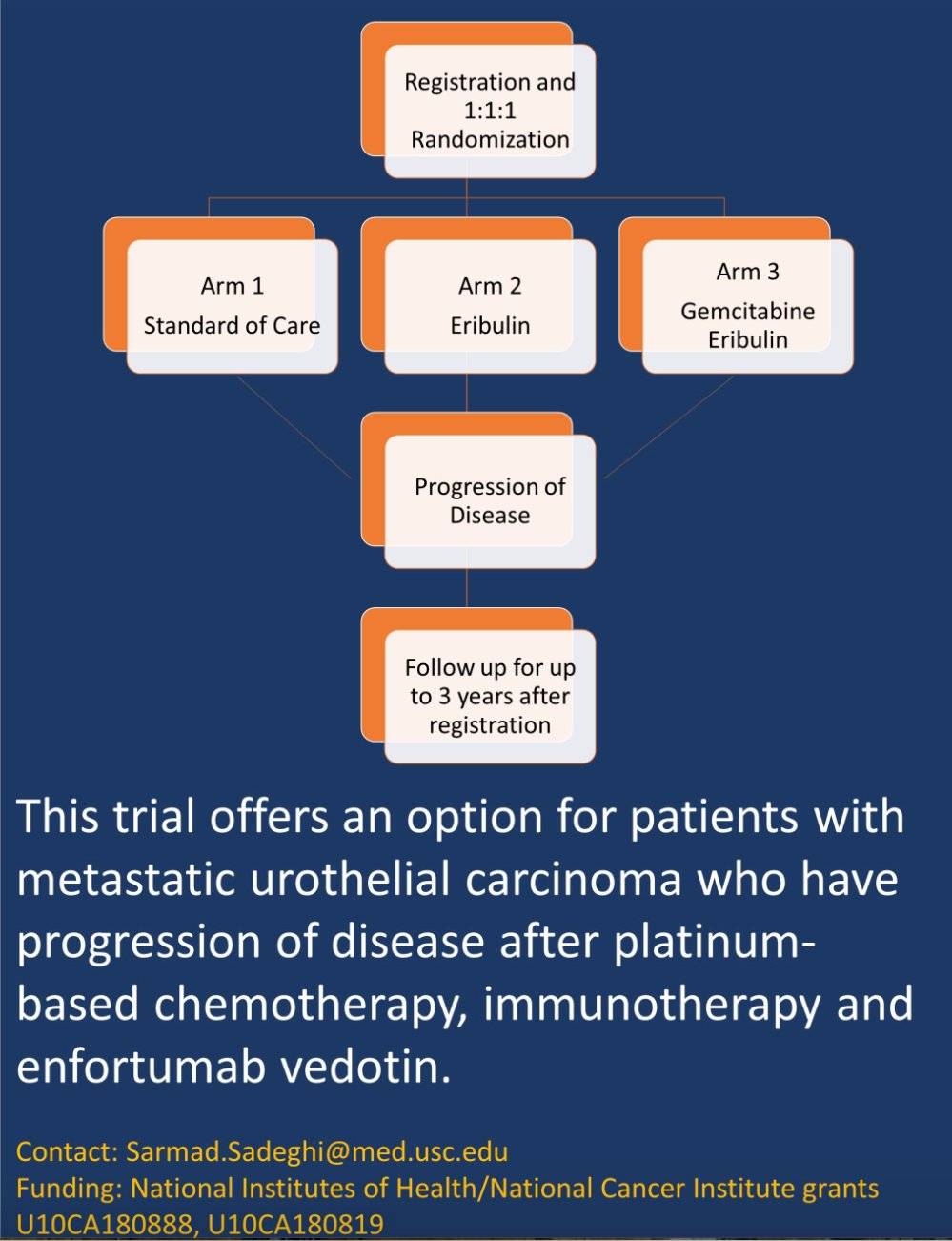
SWOG S1937 (NCT04579224) is a three-arm randomized phase II trial including patients with metastatic urothelial carcinoma and the following eligibility criteria:
- Received 1st line systemic chemotherapy, such as cisplatin-based chemo or a non-platinum regimen
- Received a PD-1/PD-L1 inhibitor or deemed ineligible for such
- Received enfortumab vedotin
This trial includes the following three study arms:
- Arm 1: Eribulin only (1.4 mg/m2 on days 1 and 8 of a 21-day cycle)
- Arm 2: Eribulin + gemcitabine (1,000 mg/m2 on days 1 and 8 of a 21-day cycle)
- Arm 3: Standard of care arm (docetaxel, paclitaxel, or gemcitabine)
This study will recruit 155 patients (140 eligible) in each arm, for a total study sample size of 465 patients. This will allow 87% power at a 1-side type 1 error of 0.0125 to detect a 50% improvement in median OS from 7 months to 10.5 months (HR: 0.67). Comparisons will be performed between each of arms 1 and 2 with arm 3. This study was activated in February 2021 and accrual is ongoing.
Presented by: Sarmad Sadeghi, MD, PhD, Associate Professor, Department of Medicine, University of Southern California, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.References:
- Sadeghi S, Groshen SG, Tsao-Wei DD, et al. Phase II California Cancer Consortium Trial of Gemcitabine-Eribulin Combination in Cisplatin-Ineligible Patients With Metastatic Urothelial Carcinoma: Final Report (NCI-9653). J Clin Oncol 2019;37(29):2682-8.


