(UroToday.com) The 2023 ASCO annual meeting included a bladder cancer session, featuring a presentation by Dr. Morgan Roupret discussing interim results of EV-104, a phase 1 study of intravesical enfortumab vedotin in patients with non-muscle invasive bladder cancer (NMIBC). A majority of patients with bladder cancer present with non-muscle invasive disease, and for patients with high-risk tumors, standard of care is resection of the tumor followed by intravesical BCG. Once patients experience recurrence and develop BCG-unresponsive disease, standard of care is radical cystectomy, which many patients are unfit for or refuse. Enfortumab vedotin is an antibody-drug conjugate (ADC) directed to Nectin-4, which is highly expressed in bladder tumors. Intravenous enfortumab vedotin has demonstrated survival benefit in patients with previously treated locally advanced or metastatic urothelial carcinoma. In preclinical models with intravesical administration, enfortumab vedotin was well tolerated and showed antitumor activity. At the 2023 ASCO annual meeting, Dr. Roupret and colleagues presented the first clinical data for intravesical administration of enfortumab vedotin in patients with NMIBC.
EV-104 (NCT05014139) is an ongoing phase 1, open-label, multicenter, dose-escalation and expansion study of intravesical enfortumab vedotin evaluating the safety, tolerability, PK, and antitumor activity of intravesical enfortumab vedotin in patients with high-risk BCG-unresponsive NMIBC with carcinoma in situ (CIS) with or without papillary disease who are ineligible for or refuse radical cystectomy. The trial design for EV-104 is as follows:
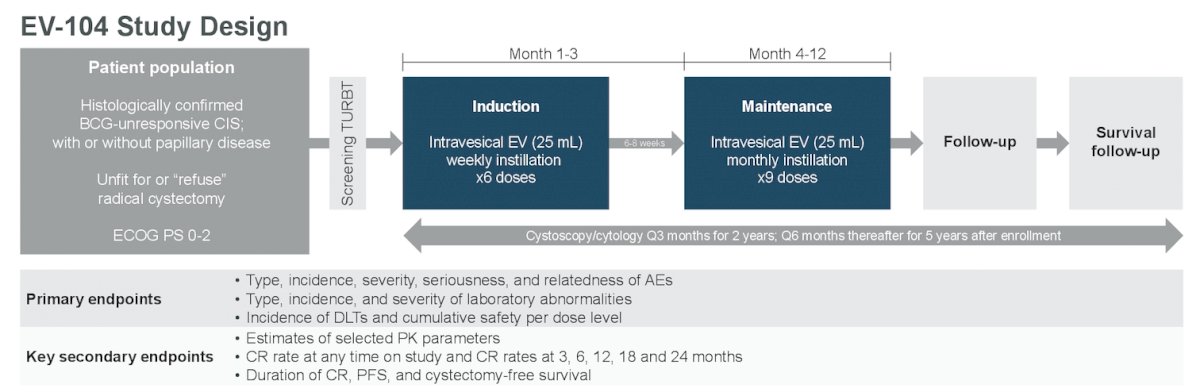
The dose escalation phase aimed to identify the maximally tolerated dose or recommended dose of intravesical enfortumab vedotin at the following dose levels: 125mg, 250mg, 500mg, and 750mg. The study design is optimized to maximize intravesical drug concentration and limit urgency with a 25 mL dose volume. Approximately 18 patients will be treated across four dosing levels during dose escalation. Patients received intravesical enfortumab vedotin weekly for 6 weeks in the induction phase, followed by 9 monthly maintenance doses.
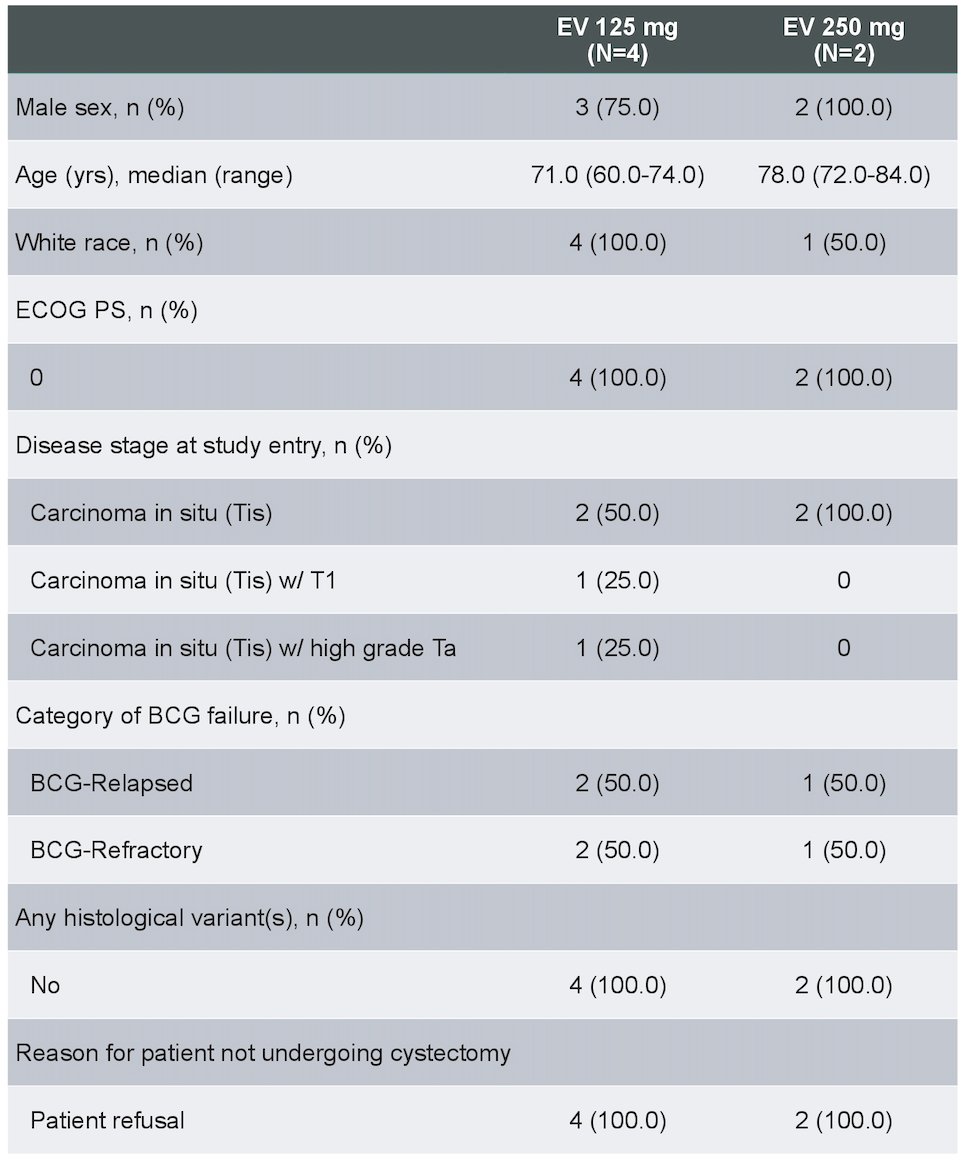
As of the data cutoff (February 10, 2023), 6 patients received intravesical enfortumab vedotin across 2 dose levels: 4 patients at 125mg completed 6 induction cycles and proceeded to maintenance; 2 patients at 250mg had received at least 3 doses of intravesical enfortumab vedotin. Key demographic and baseline disease characteristics are as follows:
Of 6 patients across doses, there were no Grade ≥3 treatment related adverse events, treatment-related symptomatic adverse events, treatment related adverse events leading to dose reduction, or discontinuation. Additionally, 4 of 6 patients (3 at 125mg and 1 at 250mg) experienced ≥1 treatment-related adverse event of Grade 1 or 2. The most common treatment related adverse events were fatigue (3 of 6) and micturition urgency (2 of 6):

As of data cutoff, no dose limiting toxicities were observed for either 125 mg or 250mg. All blood PK analyses (ADC and unconjugated MMAE) for patients at 125mg were undetectable; no PK data was available for 250mg. Of 4 patients receiving 125mg of intravesical enfortumab vedotin, 3 achieved complete response and continue in response. The fourth patient discontinued treatment due to persistent disease but remains on study:
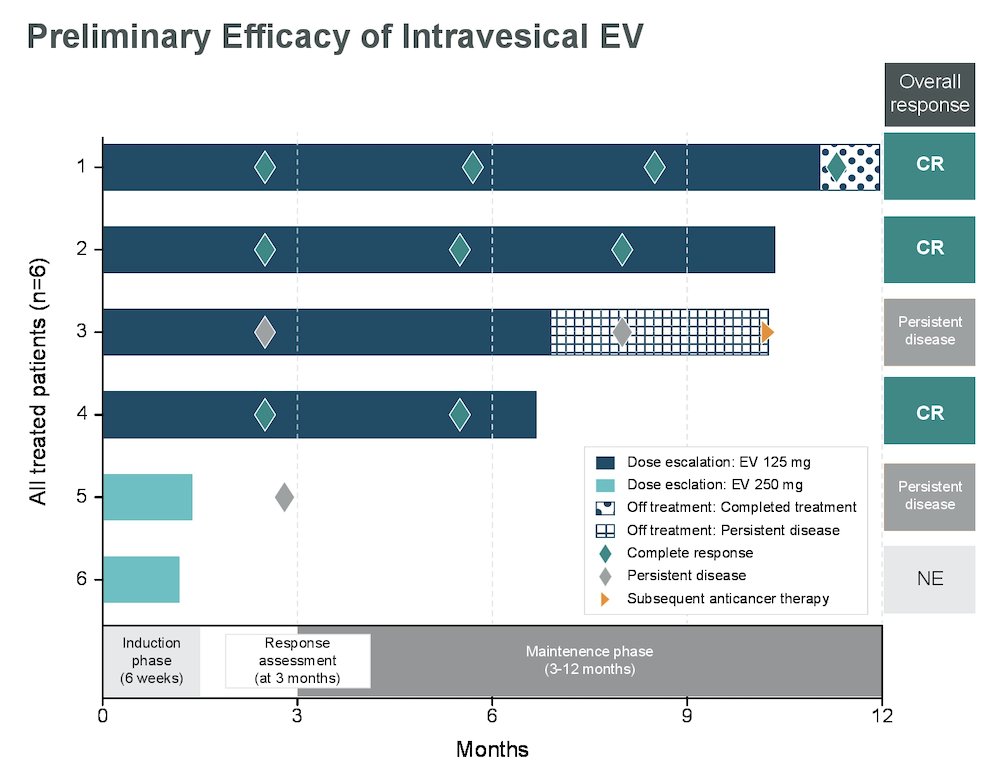
Patients at 250mg had not been evaluated for response at the data cutoff.
Dr. Roupret concluded his presentation by discussing the interim results of EV-104, a phase 1 study of intravesical enfortumab vedotin in patients with NMIBC with the following take-home messages:
- Preliminary data from EV-104 show intravesical enfortumab vedotin is well tolerated with no evidence of systemic exposure at 125mg
- Preliminary antitumor activity was observed at 125mg with 3 of 4 patients achieving a complete response
- Dose escalation is ongoing to identify the maximally tolerated dose or recommended dose for dose expansion
Presented by: Morgan Roupret, PhD, Hospital Pitie-Salpetriere, Paris, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


