(UroToday.com) The 2023 ASCO annual meeting included a bladder cancer session, featuring a trials in progress presentation by Dr. Andrea Necchi discussing KEYNOTE-905/EV-303, a phase 3 study evaluating the efficacy and safety of perioperative pembrolizumab or pembrolizumab + enfortumab vedotin for MIBC. Standard of care for MIBC is neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy + pelvic lymph node dissection, but many patients with MIBC are ineligible for cisplatin-based chemotherapy. In the phase 1b/2 KEYNOTE-869/EV-103 study, combination therapy with the PD-1 inhibitor pembrolizumab and the Nectin-4–directed antibody-drug conjugate enfortumab vedotin showed promising antitumor activity in cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma. KEYNOTE-905/EV-303 (NCT03924895) is a multicenter, randomized, open-label, phase 3 study to evaluate the efficacy and safety of perioperative pembrolizumab alone or combined with enfortumab vedotin versus radical cystectomy + pelvic lymph node dissection alone in patients with MIBC who are ineligible for or decline cisplatin-based treatment.
For this trial, patients with treatment-naive MIBC (T2-T4aN0M0 or T1-T4aN1M0) and predominantly (≥50%) urothelial histology who are cisplatin ineligible or decline cisplatin-based treatment and have an ECOG PS score of 0-2 are eligible. Cisplatin ineligibility will be defined as meeting ≥1 of the following criteria:
- Impaired renal function with calculated creatinine clearance 30-59 mL/min
- ECOG PS 2
- Grade ≥2 audiometric hearing loss per CTCAE v4.0
- NYHA class III heart failure
Patients will be randomized to three arms:
- Arm A: neoadjuvant pembrolizumab 200 mg IV every 3 weeks for ≤3 cycles followed by radical cystectomy + pelvic lymph node dissection and adjuvant pembrolizumab 200 mg IV every 3 weeks for ≤14 cycles
- Arm B: radical cystectomy + pelvic lymph node dissection followed by observation (adjuvant nivolumab permitted based on clinical indication and regulatory approval)
- Arm C: neoadjuvant enfortumab vedotin 1.25 mg/kg + pembrolizumab 200 mg IV every 3 weeks for ≤3 cycles followed by radical cystectomy + pelvic lymph node dissection and adjuvant enfortumab vedotin + pembrolizumab for ≤6 cycles and adjuvant pembrolizumab 200 mg IV every 3 weeks for ≤8 cycles
In the neoadjuvant and adjuvant phases of arm C, pembrolizumab will be administered on day 1 and enfortumab vedotin will be administered on days 1 and 8 of each cycle. Enrollment is complete for arm A, whereas patients will continue to be randomly assigned 1:1 to arm B or arm C. The trial design for KEYNOTE-905/EV-303 is as follows:
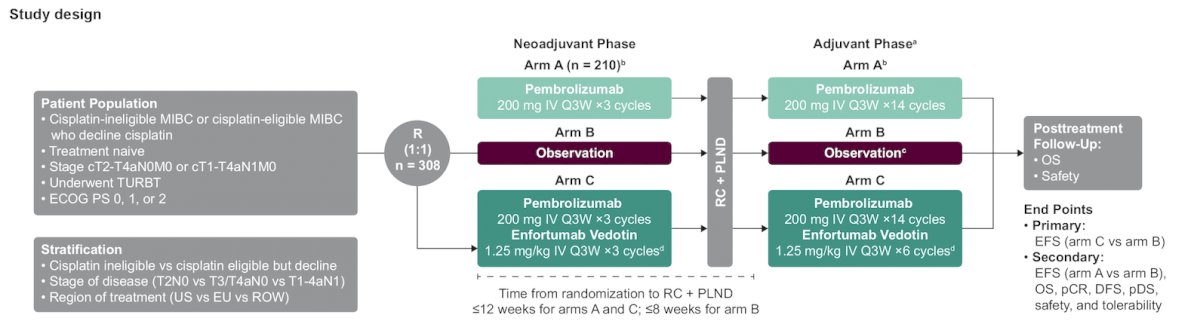
Stratification factors are tumor stage (T2N0 vs T3/T4aN0 vs T1-T4aN1), geographic region (United States vs Europe vs most of the world), and cisplatin eligibility (cisplatin ineligible or cisplatin eligible but declines treatment). The primary endpoint is event-free survival between arm B and arm C. Secondary endpoints include event-free survival (arm A vs arm B) pathologic complete response, overall survival, disease-free survival, pathologic downstaging rates, and safety.
Dr. Necchi concluded his presentation discussing KEYNOTE-905/EV-303 by highlighting that enrollment is planned for 308 patients, and recruitment is ongoing in Africa, Asia, Australia, Europe, North America, and South America:

Presented by: Andrea Necchi, MD, Vita-Salute San Raffaele University; IRCCS San Raffaele Hospital and Scientific Institute, Milan, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


