(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting included a bladder cancer session, featuring a presentation by Dr. Thomas Flaig discussing updated results for Cohort H of the EV-103 trial assessing neoadjuvant treatment with enfortumab vedotin monotherapy in cisplatin-ineligible patients with muscle-invasive bladder cancer (MIBC).
Cisplatin-ineligible patients with MIBC have no established neoadjuvant treatment options known to prolong survival before undergoing radical cystectomy and pelvic lymph node dissection, enfortumab vedotin is an antibody-drug conjugate directed to Nectin-4, which is highly expressed in urothelial cancer. In a phase 3 study, enfortumab vedotin showed improved overall survival vs standard chemotherapy and a tolerable safety profile in patients with advanced urothelial cancer previously treated with a platinum agent and a PD-1/L1 inhibitor [1]. In Cohort H of the EV-103 phase 1b/2 study, preliminary results, including pathological complete response and pathological downstaging rates, showed antitumor activity in cisplatin-ineligible patients with MIBC who received neoadjuvant enfortumab vedotin treatment. At ASCO 2023, Dr. Flaig and colleagues reported updated results, including event-free survival and subsequent cancer-related therapy.
Cohort H of the EV-103 phase 1b/2 study (NCT03288545) enrolled cisplatin-ineligible patients with MIBC (T2-T4aN0M0) and ECOG PS ≤2 who were eligible for radical cystectomy and pelvic lymph node dissection. Patients received neoadjuvant enfortumab vedotin (1.25 mg/kg) on Days 1 and 8 every 21 days for 3 cycles before undergoing radical cystectomy and pelvic lymph node dissection. The trial design for EV-103 cohort H is as follows:
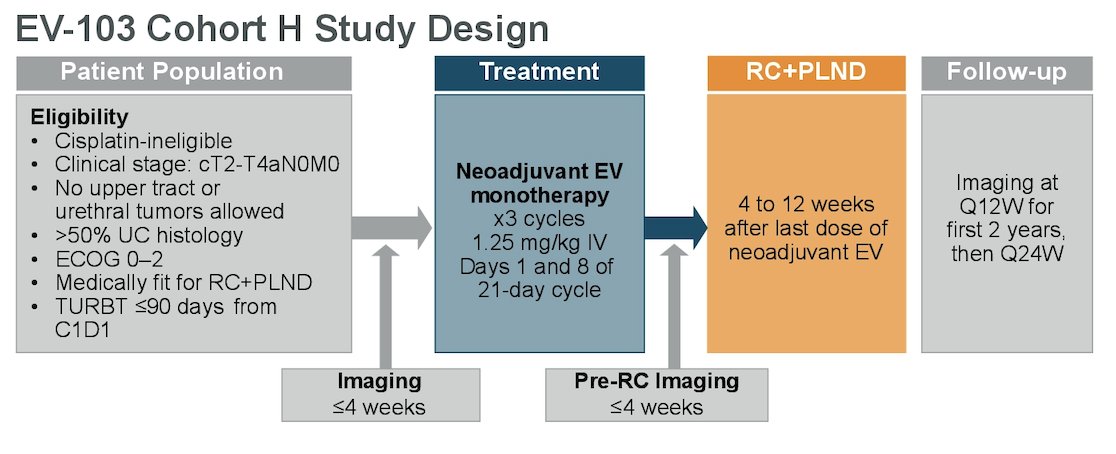
The primary endpoint was pathological complete response rate (ypT0 and N0) by central pathology review. Key secondary endpoints included pathological downstaging rate (ypT0, ypTis, ypTa, ypT1, and N0), event-free survival based on investigator assessment, and safety.
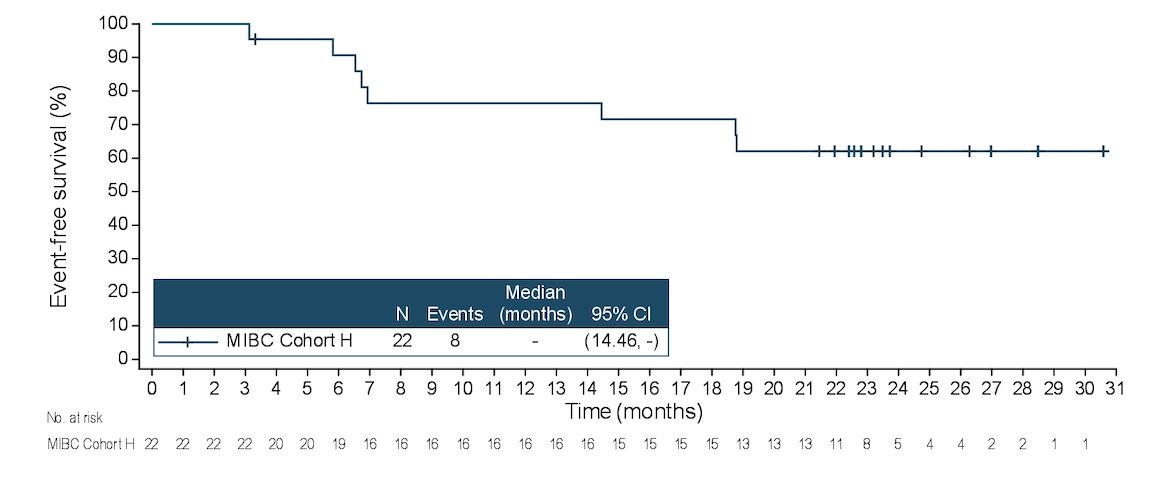
There were 22 patients (median age 74.5 years, range 56-81) enrolled and treated, including 17 patients (77.3%) remaining on study. Patients had stage cT2 (68.2%), cT3 (27.3%), or cT4 (4.5%) disease. There were 68.2% of patients that had transitional cell carcinoma only, as well as 31.8% that had a mixed histology. Overall, 86.4% of patients completed all 3 cycles of enfortumab vedotin treatment, with a median duration of enfortumab vedotin treatment of 2.1 months (range 0.7-2.3). As of the data cutoff of January 17, 2023, the pathological complete response rate was 36.4% (95% CI, 17.2, 59.3) the pathological downstaging rate was 50.0% (95% CI, 28.2, 71.8), and median event-free survival has not been reached. The event-free survival rate at 12 months was 76.4% (95% CI, 52.2, 89.4):
All patients underwent surgery with no delays due to enfortumab vedotin-related treatment-emergent adverse events. The most common enfortumab vedotin-related treatment emergent adverse events were fatigue (45.5%), dysgeusia (36.4%), and alopecia (31.8%). Overall, 18.2% of patients had grade ≥3 enfortumab vedotin-related treatment emergent adverse events, 68.2% of patients had surgery-related treatment emergent adverse events, and 31.8% patients had grade ≥3 surgery-related treatment emergent adverse events. The most common surgery-related treatment emergent adverse events were procedural pain (18.2%), anemia (13.6%), and constipation (13.6%). There were 3 patients that died due to adverse events unrelated to enfortumab vedotin treatment, with 2 patients having adverse events occurring >30 days after radical cystectomy and pelvic lymph node dissection. Finally, 36.4% of patients received subsequent cancer-related therapy, as summarized below:

Dr. Flaig concluded his presentation discussing updated results for Cohort H of the EV-103 trial assessing neoadjuvant treatment with enfortumab vedotin monotherapy in cisplatin-ineligible patients with MIBC with the following take-home messages:
- Neoadjuvant enfortumab vedotin monotherapy treatment showed promising results for antitumor activity and event-free survival with a manageable safety profile in cisplatin-ineligible patients with MIBC
- All patients were able to undergo surgery with no delays due to neoadjuvant enfortumab vedotin-related treatment emergent adverse events in this understudied population
- The observed safety profile of neoadjuvant enfortumab vedotin monotherapy in this cohort is consistent with the known adverse event profile of enfortumab vedotin in other settings
- These results support ongoing phase 2 and 3 programs in MIBC evaluating enfortumab vedotin alone or combined with pembrolizumab (EV-103 Cohort L, KEYNOTE-905, KEYNOTE-B15)
Presenter by: Thomas W. Flaig, MD, University of Colorado Cancer Center Anschutz Medical Campus, Aurora, CO
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021 Mar 25;384(12):1125-1135.


