(UroToday.com) The 2023 ASCO annual meeting included a bladder cancer session, featuring a presentation by Dr. Minas Economides discussing results of a phase 2 trial assessing long-term outcomes of pembrolizumab in combination with gemcitabine and concurrent hypofractionated radiation therapy as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder.
Trimodality therapy is a standard treatment option for muscle-invasive urothelial cancer of the bladder, which has been gaining additional prominence through well designed propensity score matched studies showing comparable outcomes to radical cystectomy.1 At the ASCO 2021 annual meeting, Dr. Economides’ group previously reported an early analysis of a phase II study of pembrolizumab added to standard trimodality therapy with maximal transurethral resection of bladder tumor (TURBT), hypofractionated radiotherapy and twice weekly gemcitabine. At the 2023 ASCO annual meeting, Dr. Economides and colleagues present updated follow up safety and efficacy results of this trial.
In this multicenter phase 2 trial, patients with clinical T2-T4aN0M0 muscle-invasive urothelial cancer of the bladder who were ineligible for or declined radical cystectomy, had ECOG performance status 0 or 1 and eGFR ≥ 30 cc/min were enrolled. Patients received pembrolizumab 200 mg IV x 1 followed by maximal TURBT and then whole bladder radiotherapy (52 Gy/20 fraction) with twice weekly gemcitabine 27 mg/m2 and pembrolizumab Q3 weeks x 3 treatments. The trial design and treatment schema are highlighted as follows:
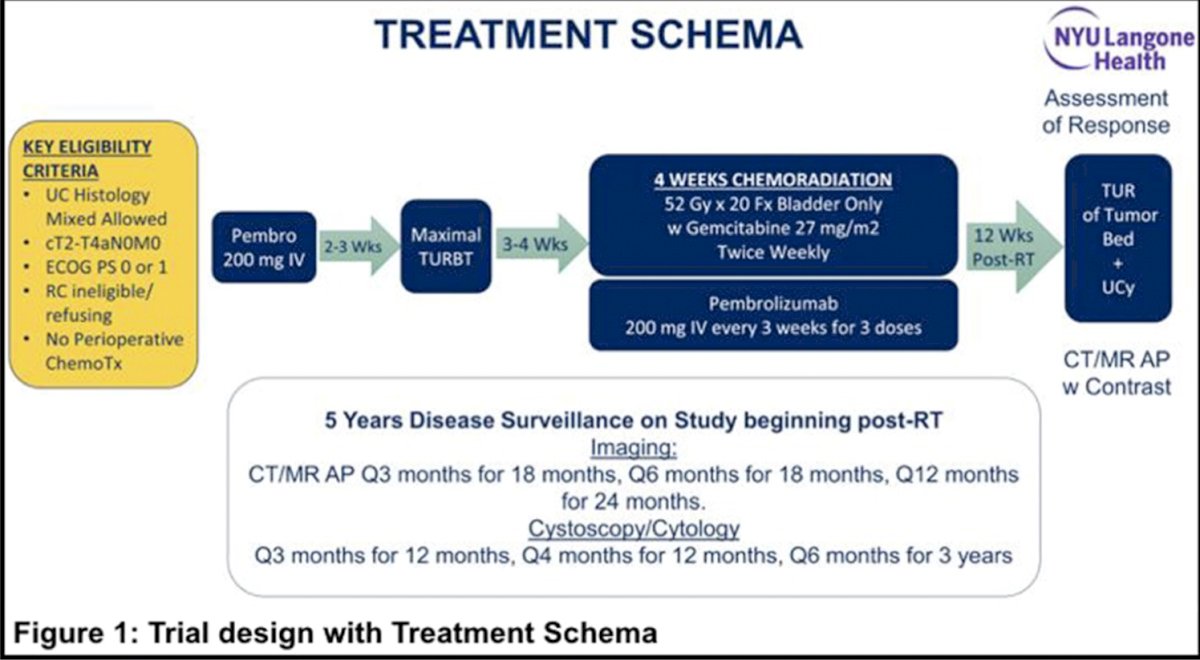
Response was assessed every 12 weeks post-radiotherapy with CT/MRI, tumor bed biopsy and cytology. The primary endpoint was 2-year bladder-intact disease-free survival (first of muscle-invasive urothelial cancer of the bladder, regional or distant metastases, cystectomy or death). The study had 85% power to detect a 20% absolute improvement in 2-year bladder-intact disease-free survival rate over 60% historical rate.1 Key secondary endpoints included safety, 12-week pathologic complete response rate, metastases-free survival and overall survival.
Between May 2016 and June 2022, 54 patients (median age 74 years, 72% male, 83% Caucasian; clinical stage: 74% T2, 22% T3 and 4% T4) were enrolled at five institutions. The baseline characteristics of patients in this trial are highlighted in the following table:

PD-L1 status was available in 43 patients, and 21 patients (49%) had modified proportion score ≥10. Forty-six (88%) patients completed all therapy, and 2% discontinued radiotherapy/gemcitabine, 6% discontinued gemcitabine, and 7% discontinued pembrolizumab, most commonly due to toxicity. There were 6 patients (11%) that underwent salvage cystectomy. As of June 2022, with a median follow-up of 23 months (range 1.6- 62.7) there were 12 (22%) tumor recurrences (3 muscle-invasive urothelial cancer of the bladder, 5 locoregional, and 4 distant). Overall, 4 patients (7%) had non-muscle invasive only recurrences. The efficacy outcomes are shown as follows:

10 patients died during the study period (18%; 3 from disease progression, 1 from treatment toxicity, and 6 from unrelated/unknown causes). Importantly, there were no new safety signals in our updated analysis: 13 patients (24%) experienced 17 adverse events that were Grade 3 or greater (cytopenia [n=7], colitis [n=5], cystitis [n=2], polyneuropathy, fatigue, hypokalemia [n=1, each]). Grade 3 or greater immune-related adverse events included 2 patients with colitis, 1 patient with polyneuropathy and 1 patient with grade 5 colonic perforation:

Given the recent literature suggesting comparable outcomes to radical cystectomy in highly selected patients, the current updated phase 2 trial results add to the current data supporting trimodality therapy.
Dr. Economides concluded this presentation discussing results of a phase 2 trial assessing long-term outcomes of pembrolizumab in combination with gemcitabine and concurrent hypofractionated radiation therapy as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder with the following take-home points:
- Trimodality therapy combined with pembrolizumab was well tolerated and continues to show promising early outcomes data
- A large phase 3 trial is underway to further explore this treatment
Presented by: Minas P. Economides, MD, New York University, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Zlotta AR, Ballas LK, Niemierko A, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: A multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023 Jun;24(6):669-681.
- Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014 Dec 1;32(34):3801-3809.


