(UroToday.com) The 2023 ASCO annual meeting included a bladder cancer session, featuring a discussant presentation by Dr. Maria De Santis discussing optimizing outcomes in upfront management of localized bladder cancer. For this presentation, Dr. De Santis discussed three abstracts including “Tumor factors and the variation in non-muscle invasive bladder cancer recurrence after transurethral resection surgery between sites: Results from the RESECT study” presented by Dr. Fortis Gaba, “Long-term outcomes of pembrolizumab in combination with gemcitabine and concurrent hypofractionated radiation therapy as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder: A multicenter phase 2 trial” presented by Dr. Minas Economides, and “FGFR3 alterations in PROOF 302: A phase III trial of infigratinib (BGJ398) as adjuvant therapy in patients with invasive urothelial carcinoma” presented by Dr. Petros Grivas.
Starting with the RESECT study, Dr. De Santis notes that there are challenges in producing guidelines, translating them into daily practice, and improving patient outcomes. As such, TURBT is an underestimated procedure with worldwide practice variations. RESECT used quality indicators to look into reasons for outcome variations and postulate that feedback and an audit may improve patient outcomes. To further highlight the importance of TURBT, Dr. De Santis quoted a 2021 UroToday interview from Dr. Ashish Kamat stating “TURBT is one of those surgeries that we tend to not recognize as probably the most critical part of the management of bladder cancer patients…if you don’t do a good TURBT, everything subsequently can…go down the wrong path, either understaging or just plain wrong treatment.” The objective of RESECT is to improve the quality of TURBT and thus improve outcomes in non-muscle invasive bladder cancer worldwide. Indeed, guidelines are based on evidence and aimed at improving patient outcomes, but adherence to guidelines and their true impact on patient outcomes are hard to measure. For example, the EAU guidelines give a strong strength rating for “In patients with tumors presumed to be at low risk and in those with small papillary recurrences (presumably Ta LG/G1) detected more than one year after previous TURBT, offer one immediate single chemotherapy instillation.” However, despite high certainty evidence, some surgeons do not request a single instillation of intravesical chemotherapy after TURBT. The primary outcomes of RESECT were four TURBT quality indicators:

In RESECT, the initial analysis of surgical and peri-operative practice showed wide variation, mean 75% (IQR 66-92) cases per site had detrusor muscle resection and 42% (IQR 17-58) had use of single instillation of intravesical chemotherapy. The median recurrence rate per site was 12% (IQR 0-22) for low grade tumors and 27% (IQR 13-42) for high grade tumors. After controlling for tumor size, number, stage and grade there was significant residual variation attributable to site (p<0.0001, intra-class correlation, 0.1). Furthermore, adjustment for sites improved the regression model from an area under the receiver operating characteristic curve of 0.66 to 0.74.
Dr. De Santis emphasized that defining the characteristics of high-quality TURBT, however, does not guarantee a better outcome. Even if all items on a checklist are performed to fulfill the criteria of high-quality TURBT, this does not mean that the procedure was done well. Thus, it is important to audit ourselves. However, RESECT showed that an audit is used in only a minority of centers:
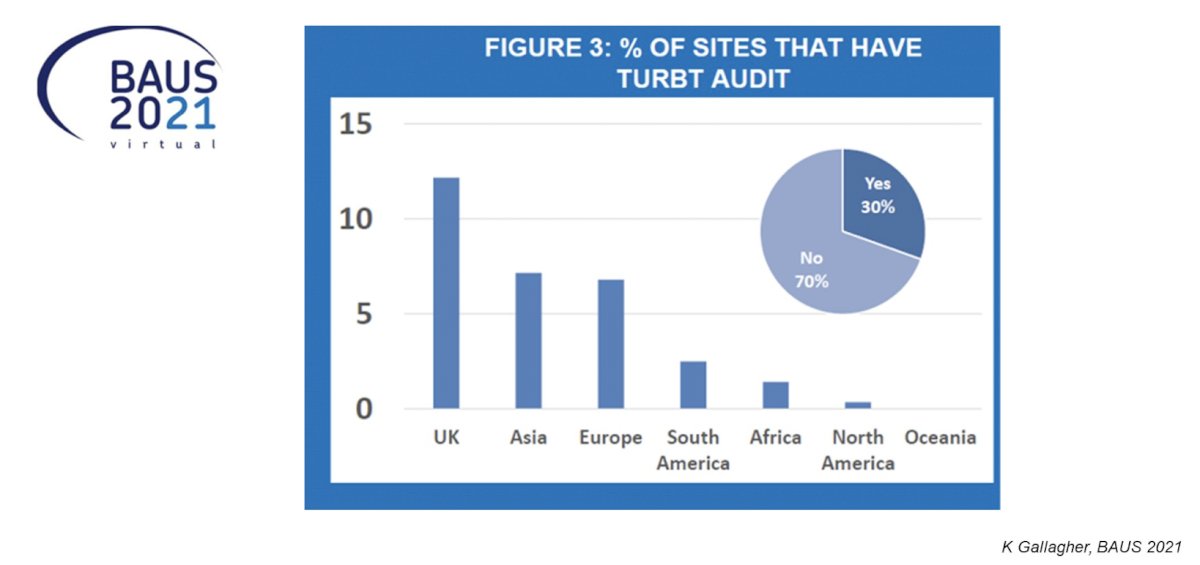
Dr. De Santis provided the following concluding statements regarding the RESECT study:
- The RESECT investigators should be commended for a huge research effort
- There is wide inter-site variation with regards to adherence to guidelines and quality indicators, with variations independent of tumor factors
- The next steps will be to inform if an audit and feedback improve TURBT surgery, reduce early recurrence rates and thus improve outcomes of bladder cancer patients
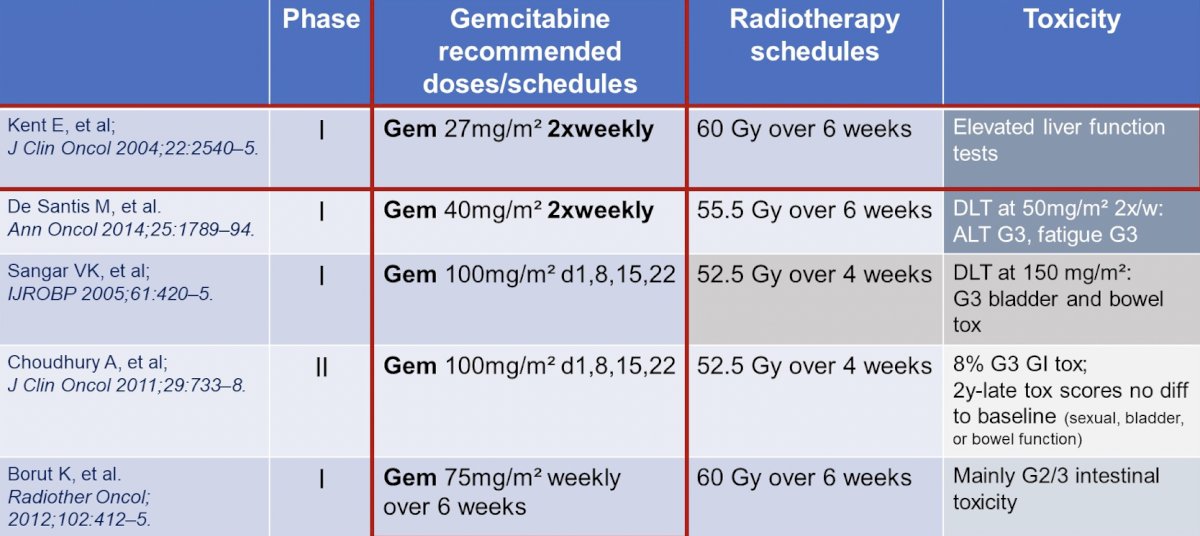
Dr. De Santis then discussed the trimodality abstract using pembrolizumab + gemcitabine for muscle invasive bladder cancer. She notes that the question in this study is: More is More? Trimodality treatment typically has included TURBT + chemotherapy + radiotherapy, however this study is evaluating if the addition of immunotherapy to these three modalities is relevant. Importantly and of note, hypofractionated radiotherapy (used in the current study) to the bladder is non-inferior to standard fractions. Chemotherapy is a radiosensitizer, with no internationally accepted standard regimens, and the addition of immunotherapy may make a difference. Data assessing hypofractionation comes from Choudhury et al1 who did a meta-analysis of the BC2001 and BCON trials, finding that a hypofractionated schedule of 55 Gy in 20 fractions is non-inferior to 64 Gy in 32 fractions with regards to both invasive locoregional control and toxicity, and is superior with regard to invasive locoregional control. As such, 55 Gy in 20 fractions should be adopted as a standard of care for bladder preservation in patients with locally advanced bladder cancer.
With regards to chemotherapy as a radiosensitizer, although there is no internationally accepted standard, several trials have assessed various agents:

Historically, there have been no comparative trials between chemotherapy regimens + radiotherapy, however both KEYNOTE-992 and NRG/SWOG 1806 are trials allowing chemotherapy as a radiosensitizer by investigator choice (cisplatin, 5FU/MMC, gemcitabine), with various radiotherapy schedules:
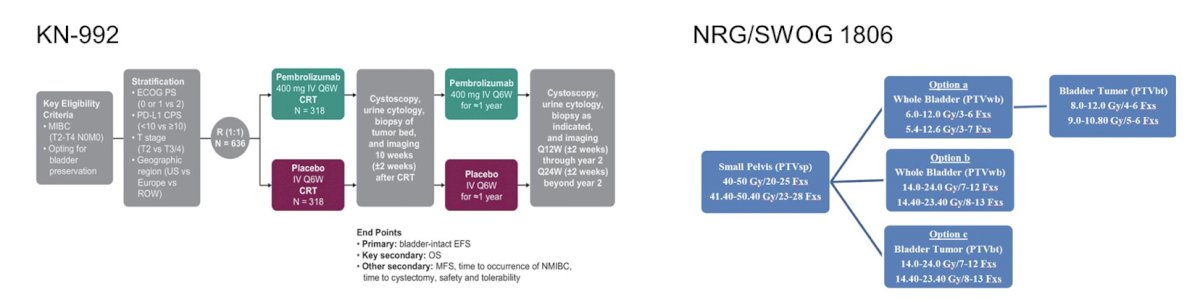
Whether immunotherapy + hypofractionation is making a difference is being assessed in several trials. However, there have been some early safety concerns. In the PLUMMB trial (36 Gy/6 weekly fractions + pembrolizumab), this trial was stopped early secondary to >= Grade 3 GU toxicity. Another trial assessing 50 Gy/20 fractions + atezolizumab + gemcitabine 100 mg/m2 weekly was also stopped early due to grade 3 adverse events/GI toxicity
For the current trial, 46 (88%) patients completed all therapy, and 2% discontinued radiotherapy/gemcitabine, 6% discontinued gemcitabine, and 7% discontinued pembrolizumab, most commonly due to toxicity. There were 6 patients (11%) that underwent salvage cystectomy. As of June 2022, with a median follow-up of 23 months (range 1.6- 62.7) there were 12 (22%) tumor recurrences (3 muscle-invasive urothelial cancer of the bladder, 5 locoregional, and 4 distant). Overall, 4 patients (7%) had non-muscle invasive only recurrences. Dr. De Santis provided the following table highlighting the 2021 and 2023 outcomes, in context with the literature:

Additionally, there were no new safety signals in the updated analysis: 13 patients (24%) experienced 17 adverse events that were Grade 3 or greater (cytopenia [n=7], colitis [n=5], cystitis [n=2], polyneuropathy, fatigue, hypokalemia [n=1, each]). Grade 3 or greater immune-related adverse events included 2 patients with colitis, 1 patient with polyneuropathy and 1 patient with grade 5 colonic perforation.
Dr. De Santis provided the following concluding statements regarding this phase 2 trimodality therapy + immunotherapy trial:
- The addition of pembrolizumab to gemcitabine 27 mg/m2 twice weekly + hypofractionated radiotherapy of the bladder seems to be safe enough to support the use in larger trials
- Toxicity is manageable and efficacy is in line with published date on various other regimens
- Based on the current data, there is no change of standard trimodality treatment practice
- Adjuvant immunotherapy is approved for high risk patients after radical cystectomy, however its benefit as part of tri- or as part of tetramodality therapy is still unclear and under investigation in large scale trials
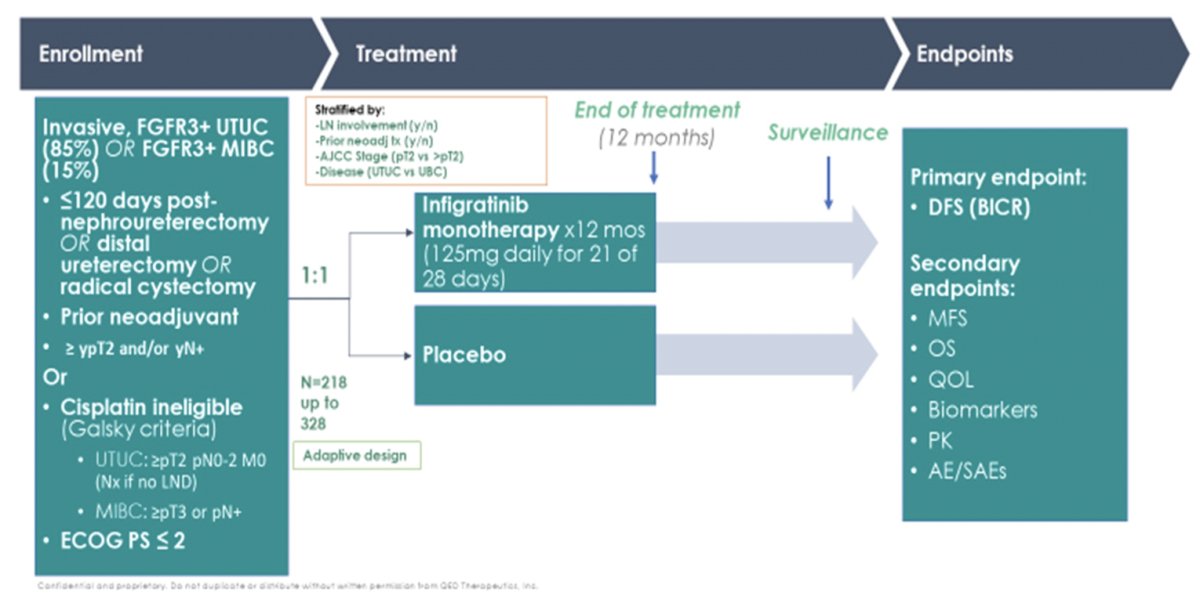
The third abstract Dr. De Santis discussed was the FGFR3 alterations from PROOF 302. Indeed, FGFR alterations are prevalent across all cancer types, playing a key role in the development of several cancers:

Previous work has shown that most FGFR alterations in urothelial carcinoma are activating mutations of S249C, R248C, Y373C. Interestingly, FGFR3 alterations are more frequently associated with upper tract urothelial carcinoma (35.6-40%) rather than bladder urothelial carcinoma (21.6-26%). Infigratinib has higher specificity for FGFR3 compared with erdafitinib for patients with the more “FGFR3-driven” upper tract tumors, thus the rational for the PROOF-302 enrichment strategy. The trial design for PROOF-302 is as follows:
The FGFR 1-4 alteration breakdown among 617 patients in PROOF 302 is as follows:
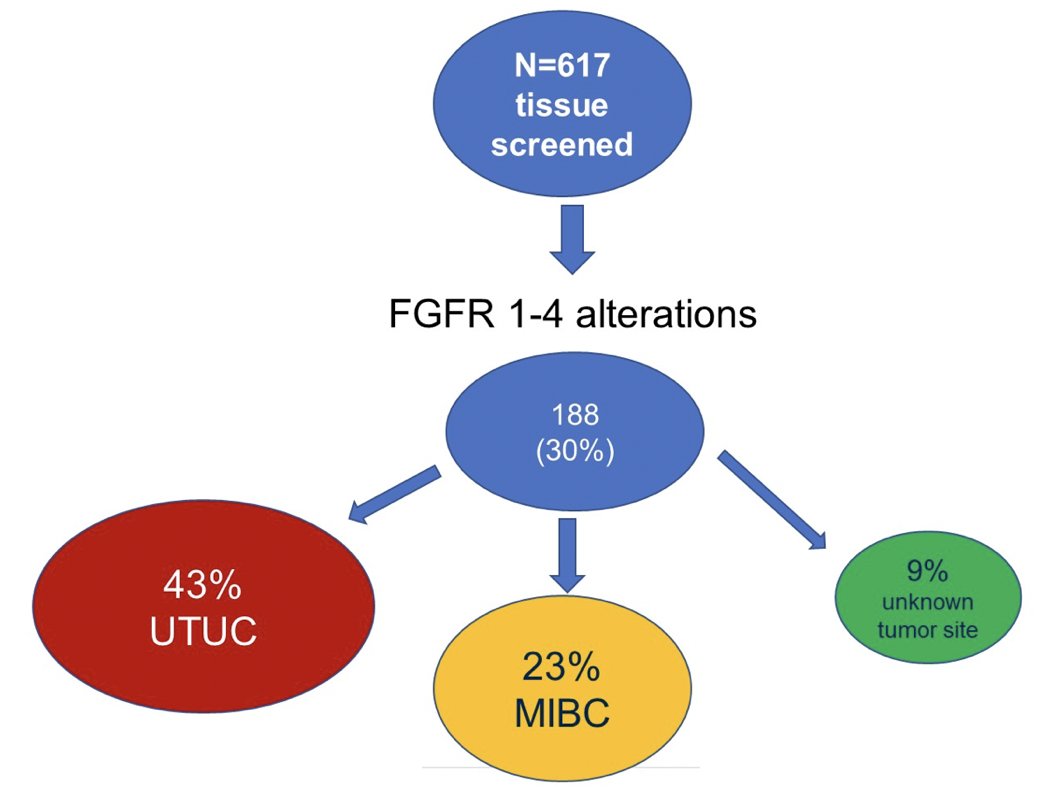
Unfortunately, secondary to the relatively lower than expected prevalence of FGFR3 alterations PROOF 302 was stopped early. Dr. De Santis provided the following concluding statements regarding the PROOF 302 trial:
- The prevalence of FGFR3 alterations in upper tract urothelial carcinoma and bladder urothelial carcinoma is slightly lower in PROOF 302 than expected in the literature. PROOF 302 stopped early, which is a missed opportunity to adequately test an adjuvant FGFR inhibitor in urothelial carcinomas with FGFR3 alterations
- To date, there is a rocky history of adjuvant trials for high risk urothelial carcinoma
- Adjuvant trials testing chemotherapy for bladder urothelial carcinoma patients have been challenging in the past
- However, adjuvant trials testing immunotherapy for bladder urothelial carcinoma were completed in a timely fashion, and nivolumab is now approved
- For pure upper tract urothelial carcinoma, so far only one randomized controlled trial testing adjuvant chemotherapy (POUT-trial positive for DFS2) has been published. Unfortunately, the benefit of adjuvant infigratinib for urothelial carcinoma patients (upper tract enriched) with FGRF4 alterations will not be answered by PROOF 302
Presented by: Maria De Santis, MD, Charité Universitätsmedizin Berlin, Berlin, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Choudhury A, Porta N, Hall E, et al. Hypofractionated radiotherapy in locally advanced bladder cancer: An individual patient data meta-analysis of the BC2001 and BCON trials. Lancet Oncol. 2021 Feb;22(2):246-255.
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomized controlled trial. Lancet 2020 Apr 18;395(10232):1268-1277.


