(UroToday.com) Tislelizumab is a humanized IgG4 anti–PD-1 monoclonal antibody specifically designed to minimize binding to FcγR on macrophages. It is produced by Novartis.
The goal of this study was to evaluate the efficacy and safety of tislelizumab combined with gemcitabine and cisplatin as neoadjuvant therapy for patients (pts) with clinical T2-T4aN0M0 (cT2-T4aN0M0) muscle-invasive bladder urothelial cancer (MIBC). They note that at predefined interim analysis, the study had met the first stage aim – which enable them to continue the study. Herein, they report the results of the predefined final analysis.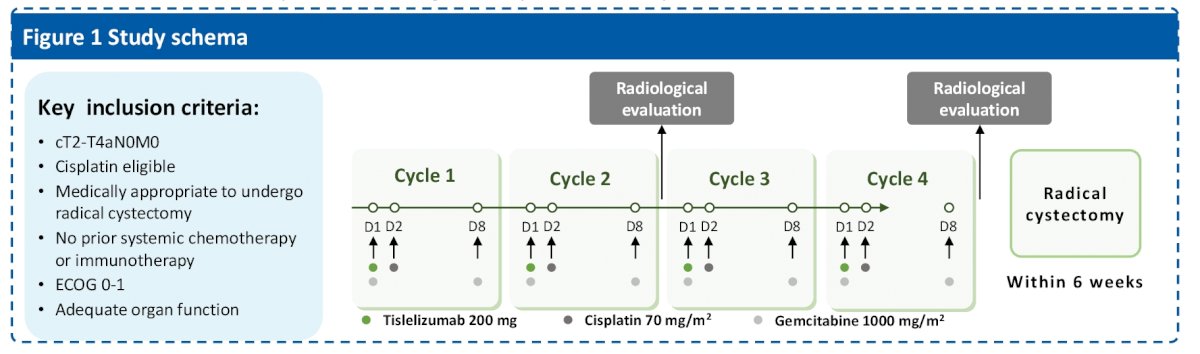
This was a phase II study that enrolled cisplatin-eligible patients with localized MIBC. Eligible patients received tislelizumab 200 mg in day 1 (D1), cisplatin 70 mg/m2 D2, and gemcitabine 1000 mg/m2 D1 and D8 every 21 days for four cycles. Radical cystectomy (RC) was performed within 6 weeks after last treatment.
Since they had radical cystectomy, the primary end point was pathologic complete response (pCR, pT0N0M0). Secondary end points were pathologic downstaging (≤pT1N0M0) (pDS), EFS, OS and safety.
At the data cut off time of 14th Dec 2022, 65 pts (38 cT2, 21 cT3, and 6 cT4a) had completed neoadjuvant therapy, with median age of 64 (44-75) years.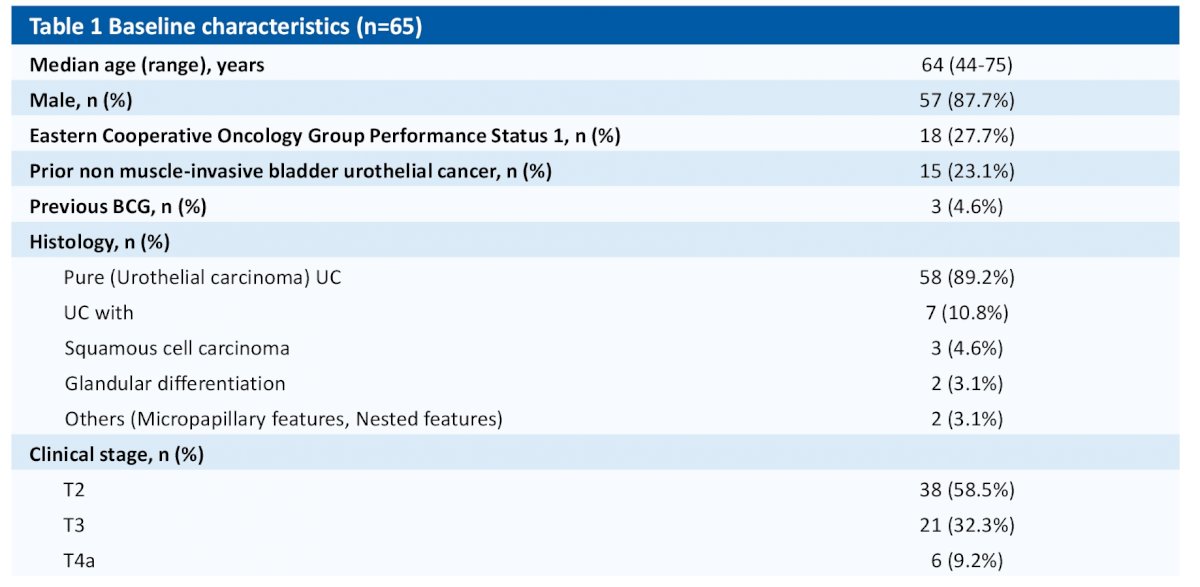
Treatment intensity of the neoadjuvant therapy is summarized below:

The majority of patients, >85% received all 4 cycles of Gem/Cis and tislelizumab, suggesting it was well tolerated.
Among these 65, patients, 57 underwent cystectomy and 8 patients declined RC. Median time from last dose to RC was 33 days (range 4-93). Surgery characteristics/details below: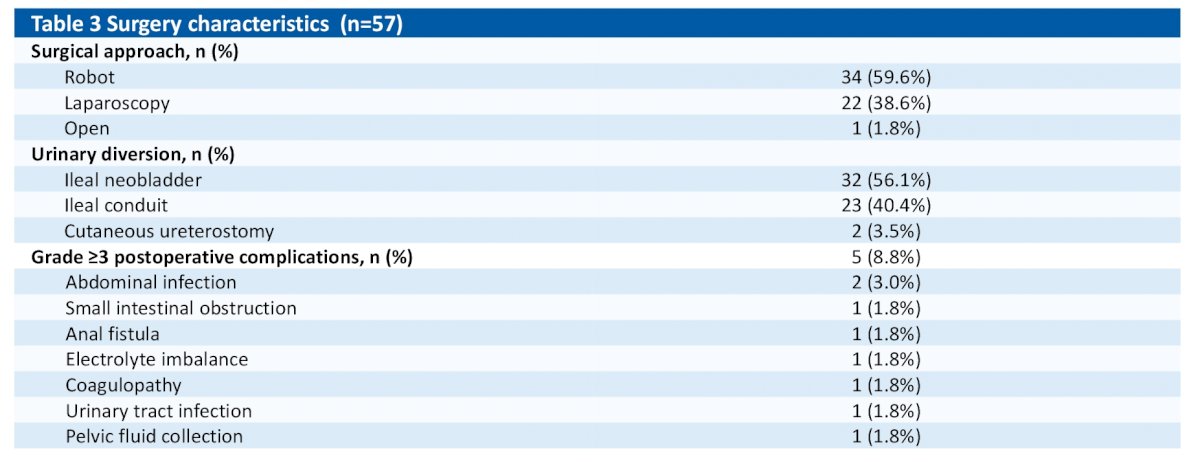
Among 57 efficacy evaluable patients,, 29 patients achieved pCR (50.9%), and 43 achieved pathologic downstaging. 27 patients achieved pCR in the first 55 evaluable patients, which met this statistical superiority criteria. three patients (1 cT2 and 2 cT3) achieved pT0 at RC, but were pathological N1.
Clavien-Dindo grade ≥3 postoperative complications occurred in 7.9% pts. Most common neoadjuvant therapy related AEs of any grade were hematologic toxicities (88.9%), nausea (77.8%), and vomiting (50.8%). Grade ≥3 neoadjuvant therapy related AEs occurred in 60.3% pts, which were hematologic toxicities (58.7%) in most. The frequently occurring immune-related AEs (irAEs) (>5%) included dizziness (14.3%), fatigue (12.7%), ASL/ALT increased (7.9%), rash (7.9%), pruritus (7.9%), and GGT increased (6.3%). Grade ≥3 irAEs occurred in 2 pts (pneumonia, n=1; cardiomyopathy, n=1).
Based on this final analysis, the authors note that the study met its primary endpoint and should be considered a positive trial. However, since it was a phase II trial, the focus was mainly safety and tolerability.
They note that the combination of Neoadjuvant tislelizumab combined with gemcitabine and cisplatin showed promising anti-tumor activity with high pCR and adequate tolerance in MIBC patients.
However, I would note that the pCR rate isn’t that much higher than reported studies of NAC cisplatin based chemotherapy alone – so the added toxicity should be weighed against the potential minimal additional benefit. Head to head comparisons would be needed to establish its incremental benefit.
Clinical trial information: ChiCTR2000037670.
Presented by: Tianxin Lin, Department of Urology, Sun Yat-sen Memorial Hospital, Sun Yat-sen UniversityWritten by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis, @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


