(UroToday.com) Dr. Scott Tagawa presents on the efficacy of sacituzumab govitecan (SG) in locally advanced (LA) or metastatic urothelial cancer (mUC) by trophoblast cell surface antigen 2 (Trop-2) expression.
He first laid the groundwork by noting that patients with metastatic urothelial carcinoma have poor outcomes, with an estimated 5-year overall survival (OS) rate of 14%. Patients with locally advanced from metastatic urothelial carcinoma who progressed after platinum based and checkpoint inhibitor therapies have limited options and an overall poor prognosis, emphasizing the need for new treatments.
Trop-2 is a transmembrane glycoprotein with elevated expression in many cancers, including UC.
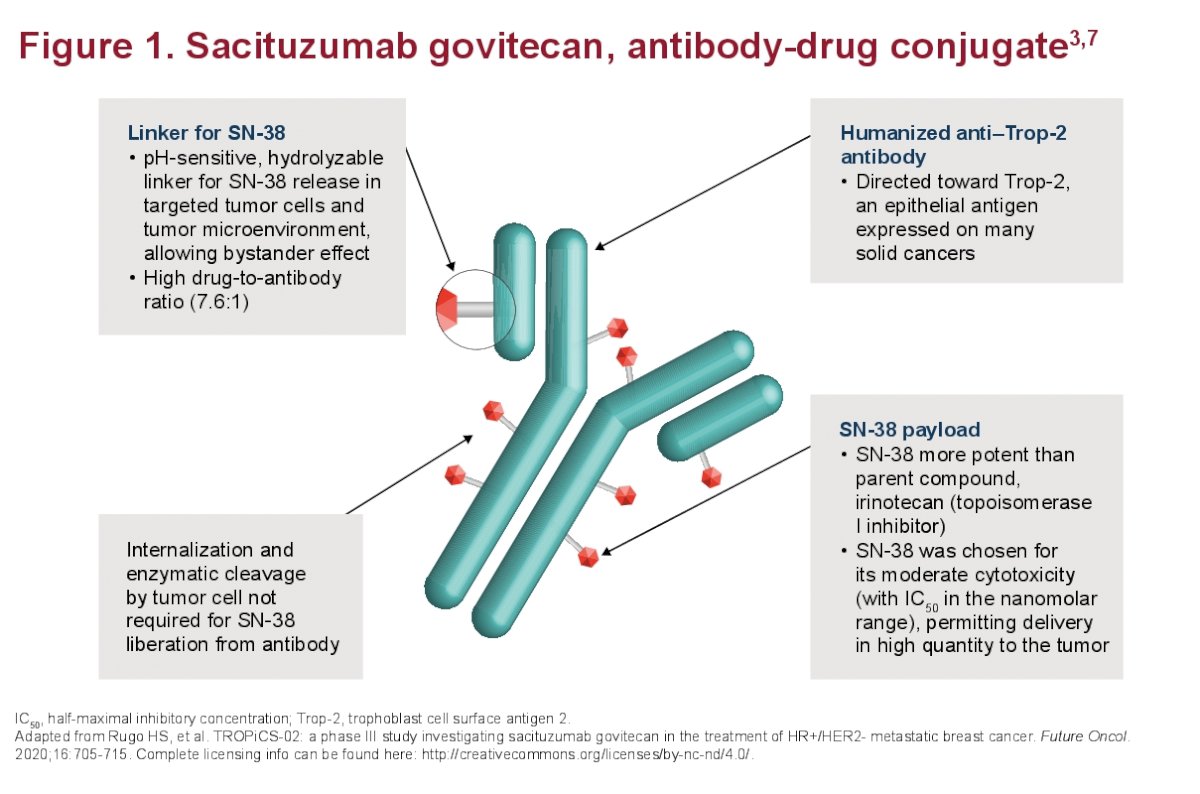
SG is a Trop-2–directed antibody-drug conjugate that received accelerated FDA approval. It is currently indicated for patients with locally advanced or unresectable metastatic UC who failed prior platinum based therapy (PT) and a checkpoint inhibitor (CPI). SG demonstrated significant antitumor activity in the three different TROPHY-U-01 mUC cohorts:
- Cohort 1 (prior platinum based therapy), 28% objective response rate (ORR)
- Cohort 2 (prior CPI), 32% ORR
- Cohort 3 (prior PT and CPI), 41% ORR.
Herein, they re-assess efficacy outcomes in Cohorts 1-3 stratified by Trop-2 archival tumor expression. Since Trop-2 is the target of the ADC, the rationale for testing it is evident.
In the original study, patients who were previously treated (platinum therapy [C3], checkpoint inhibitor [C2], or both [C1]) locally advanced or metastic Urothelial carcinoma received SG (10 mg/kg IV) on D1 and D8 of 21-D cycles. Patients in cohort 3 also received pembrolizumab (200 mg) on D1 of 21-D cycles.
For the purposes of this analysis, archival tumor samples collected at enrollment were assessed for Trop-2 protein expression using the SP295 anti–Trop-2 antibody by IHC.
At data cutoff, 192 patients were enrolled in Cohorts 1-3. Of these, 144 pts (75%) had samples evaluable for Trop-2 prevalence and 139 pts (72%) were evaluable for efficacy analysis by Trop-2 expressions. He noted that baseline characteristics for patients with evaluable samples were consistent with the overall population – and as these were randomized, were relatively similar between cohorts.
Trop-2 expression:
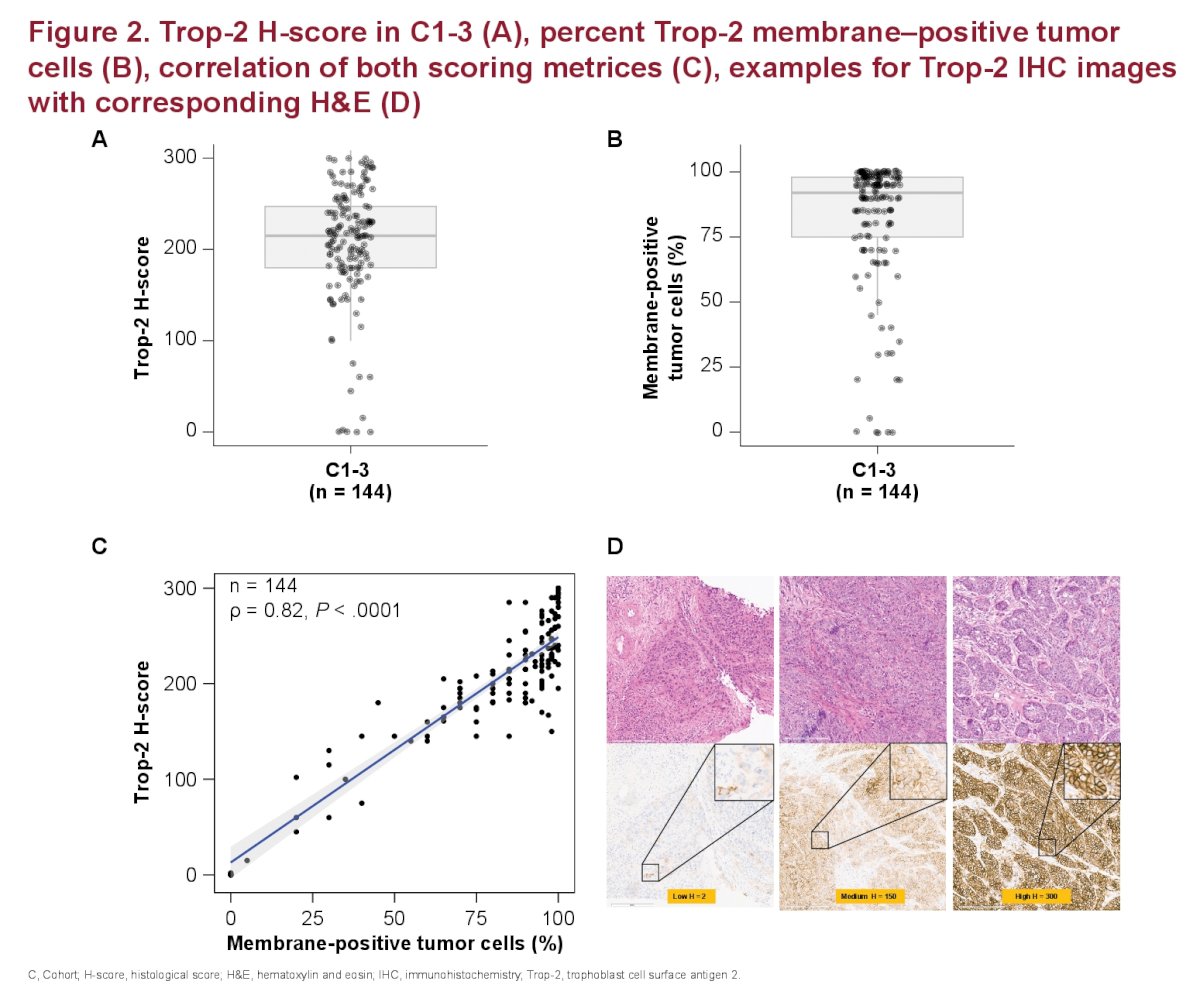
- Trop-2 was highly expressed in tumor tissue samples from patients enrolled in the TROPHY study, in line with published data
- Median (IQR) Trop-2 H-score and % of membrane-positive tumor cells for evaluable pt samples were 215 (180-247) and 92% (75-98), respectively
- These readouts were highly correlated (ρ=0.82, P<0.001).
Despite the trop-2 level readouts being in line with published data, there was no association between overall response rates (ORR) and Tro-2 expression in all groups: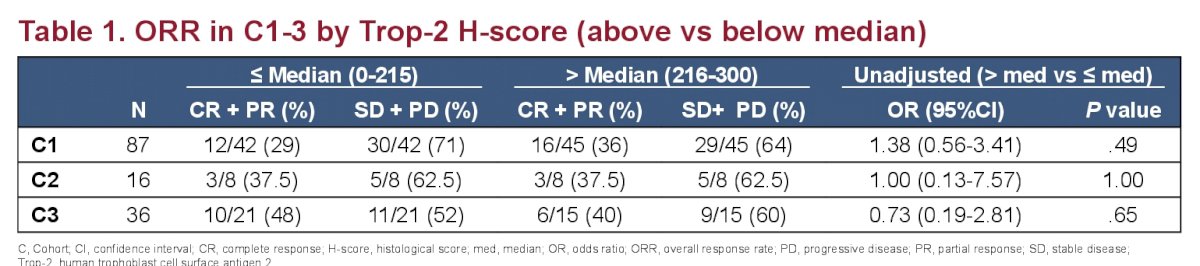
With regard to PFS and OS, there is no statistically significant relationship between Trop-2 and PFS four OS regardless of how Trop-2 was categorized.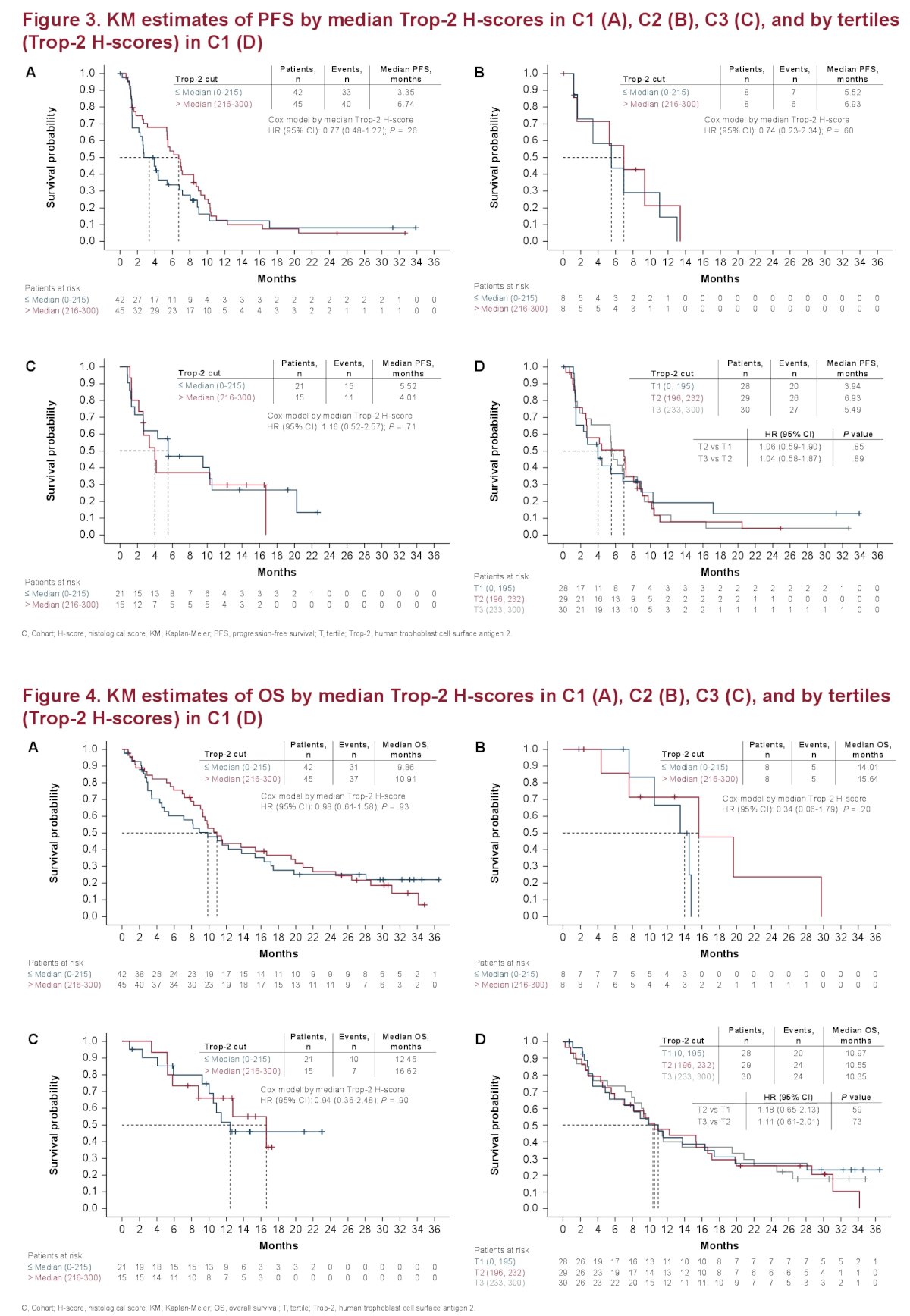
As such, while SG is meant to target Trop-2 expression, it would appear that SG activity is independent of Trop-2 expression.
Their conclusion was the following:
- Trop-2 protein was highly expressed across cohorts, supporting prior Trop-2 expression analyses in UC. This would explain its utility as a target for SG
- Similar clinical outcomes were observed among patients with samples that were below and above median Trop-2 H-score. These results suggest that SG activity may be independent of Trop-2 expression in UC.
The authors note that additional studies are needed to confirm these results. In the meantime, unfortunately, Trop-2 levels are not useful as a biomarker of SG response.
Clinical trial information: NCT03547973.
Presented by: Scott T. Tagawa, MD, FASCO, FACP, MS, Weill Cornell Medicine, New York, NYWritten by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis, @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


