(UroToday.com) Dr. Patrizia Giannatempo presents interim results from the phase 2 ARCADIA trial, evaluating cabozantinib (CABO) plus durvalumab (DURVA) in patients (pts) with advanced urothelial carcinoma (UC) or non-UC variant histologies (VH) after platinum chemotherapy.
She first began with the rationale for the combination of a TKI with a checkpoint inhibitor. Cabozantinib, which is a multitargeted receptor tyrosine kinase inhibitor (TKI), may have immunomodulatory effects that can be synergistic with checkpoint inhibitors such as durvalumab.
Dr. Giannatempo and her colleagues, in this phase II study, evaluate the combination of CABO and DURVA in patients with advanced urothelial and non-urothelial carcinoma of the bladder (NCT03824691). Herein they present the interim analysis. This is not the final analysis, which will have to be presented later.
In brief, they included patients with either urothelial and non-urothelial carcinoma of the bladder who recurred of progressed after at least one line of platinum-based chemotherapy for metastatic disease. They were treated with Cabo 40 mg daily and DURVA 1500 mg IV q28 days until disease progression by RECIST 1.1 criteria or unacceptable toxicity.
- Key inclusion criteria were ECOG-PS 0-1 and adequate organ function.
The primary endpoint of the study was overall survival (OS).
Secondary endpoints included safety (CTCAE v.4.03), objective response-rate (ORR), duration of response (DoR), progression-free survival (PFS).
Between September 2019 and February 10, 2023, the ARCADIA study enrolled 63 patients. They waited until at least one baseline tumor assessment was done in 58 patients before running the interim analysis.
- Median follow-up was 23.5 months
Full demographics are seen below:
It should be pointed out that 20 pts (34%) had a pure/predominant non-UC VH - including 9 (45%) with squamous differentiation/sarcomatoid tumor, 5 (25%) an adenocarcinoma, 4 (20%) a small-cell neuroendocrine tumor, 1 (5%) a clear-cell tumor, and 1 a nested VH (5%).
Some patients (N=11, 19%) were heavily pretreated and had received ≥2 prior systemic anticancer therapies.
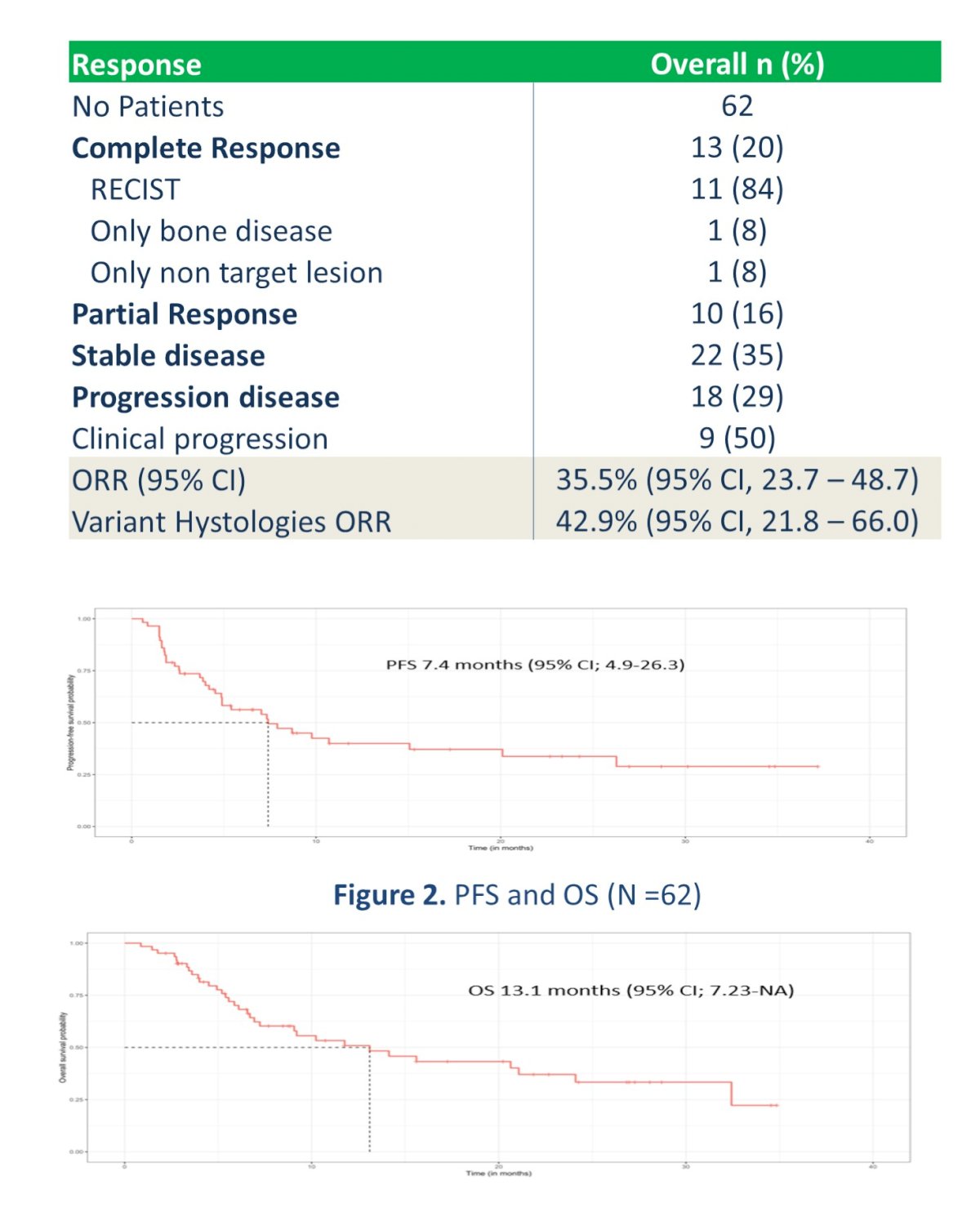
In 58 response-evaluable patients with at least one scan, 12 (20%) had a complete responses (CR) and 11 had a partial responses (PR). This resulted in an ORR of 39.7% while disease control rate was 69%. These are excellent outcomes in this disease setting.
Importantly, in the cohort of patients with variant histology, the ORR was 45% (95%CI: 23.1-68.5). Median PFS was 7.6 mos (95%CI: 4.6-13-6 mos) and median OS was 11.6 (95% CI 6.8-20.3 months). This is a less studied cohort and often not as responsive to the same therapies as pure UC. As such, their reporting on the subgroup is important for treatment selection and sequencing.
Median exposure of treatment was 4.8 months. 35 pts (55.5%) of 63 pts had all-grade treatment-related adverse events (AE) and 4 pts (6.3%) reported grade 3 (treatment related) AE with no grade 3-5 events. Dose-reductions of CABO were needed in 25 pts (39%). No treatment-related deaths were reported.
Based on this interim results presentation, they concluded that the cabo/durva combination had promising preliminary activity with a manageable safety profile in this patient population. Importantly, this included patients with non-UC VH as well as urothelial histology.
We will await more mature results with longer follow-up in the future. Ultimately, OS is the primary endpoint.
Clinical trial information: NCT03824691.
Presented by: Patrizia Giannatempo, MD, IRCCS Foundation National Cancer Institute, Milan, ItalyWritten by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis, @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


