(UroToday.com) The 2023 ASCO annual meeting included a late-breaking abstract session, featuring a discussant presentation by Dr. Daniel Petrylak discussing the phase 3 THOR study, a randomized phase 3 trial assessing erdafitinib versus chemotherapy in patients with advanced or metastatic urothelial cancer with select fibroblast growth factor receptor (FGFR) alterations. Metastatic urothelial cancer is responsible for 16,710 deaths in the United States in 2023, and approximately 212,000 deaths globally. Currently, the standard of care treatments are as follows:
- First-line cisplatin eligible: gemcitabine + cisplatin or MVAC followed by avelumab maintenance
- Cisplatin ineligible: (i) gemcitabine + cisplatin followed by avelumab maintenance, (ii) enfortumab/pembrolizumab
- Second line checkpoint therapy: (i) pembrolizumab, (ii) avelumab, (iii) nivolumab
- Third line: (i) enfortumab vedotin, (ii) sacituzumab govitecan, (iii) erdafitinib
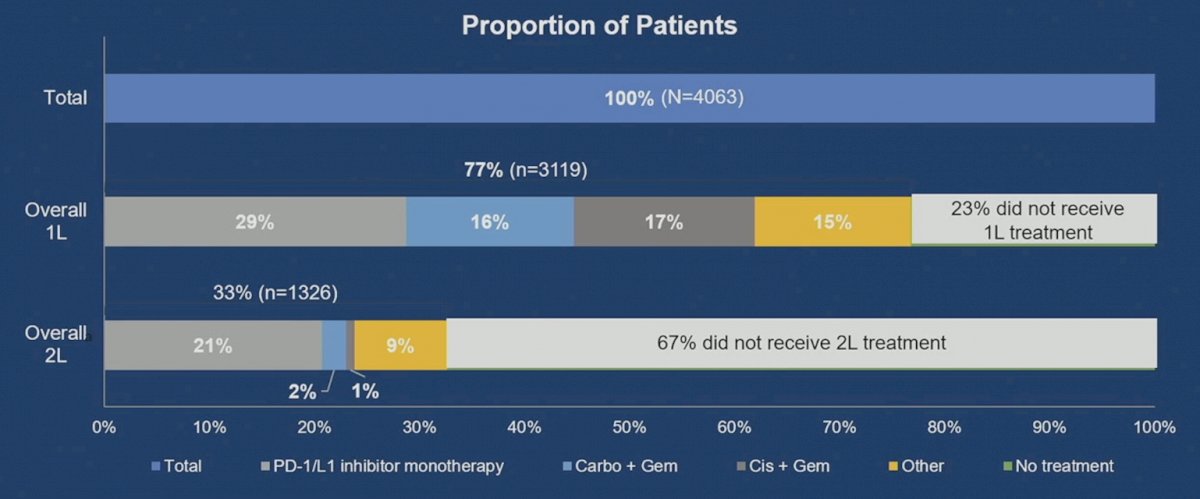
Dr. Petrylak emphasized that one-fourth of advanced urothelial carcinoma patients do not receive first line treatment and two-thirds do not receive second line treatment:
Biomarker testing continues to be more important and gaining prominence in the treatment of metastatic urothelial carcinoma. In a study by Nimgaonkar et al. [1] in JAMA Oncology in 2022, 761 patients received post platinum therapy from April 1, 2019 to September 1, 2021 of which 343 patients (41%) underwent FGFR testing. Among these patients 305 had tissue-based testing and 74 patients had blood-based testing. Ultimately 71 patients (20.7%) had a susceptible FGFR alteration and 30 patients (30%) received erdafitinib. As such, Dr. Petrylak states that based on this data, and data present from THOR cohort 1: all patients with metastatic urothelial carcinoma need to be tested for FGFR3/2alt mutations.
FGFR3 is a fibroblast growth factor receptor, which is a membrane based TKR involved in cellular proliferation, differentiation, and steroid biosynthesis. There are four distinct FGFR subtypes and FGFR mutations and overexpression have been implicated in urothelial cancer. On April 12, 2019, the FDA granted accelerated approval to the FGFR inhibitor erdafitinib for locally advanced and metastatic urothelial carcinoma with an FGFR2 or FGFR3 alteration, that has progressed during or after platinum chemotherapy. FGFR inhibitors and anti-FGFR antibody drug conjugates are currently in ongoing and upcoming trials in advanced urothelial carcinoma.
Dr. Petrylak notes that Ta tumors express FGFR alterations in up to 90% of specimens, and expression is lower in tumors T2 or higher (10-20%). Mutations can occur in the extracellular or transmembrane domain of the receptor, and lead to ligand independent dimerization of the receptor, which is a similar mechanism for fusion mutations.
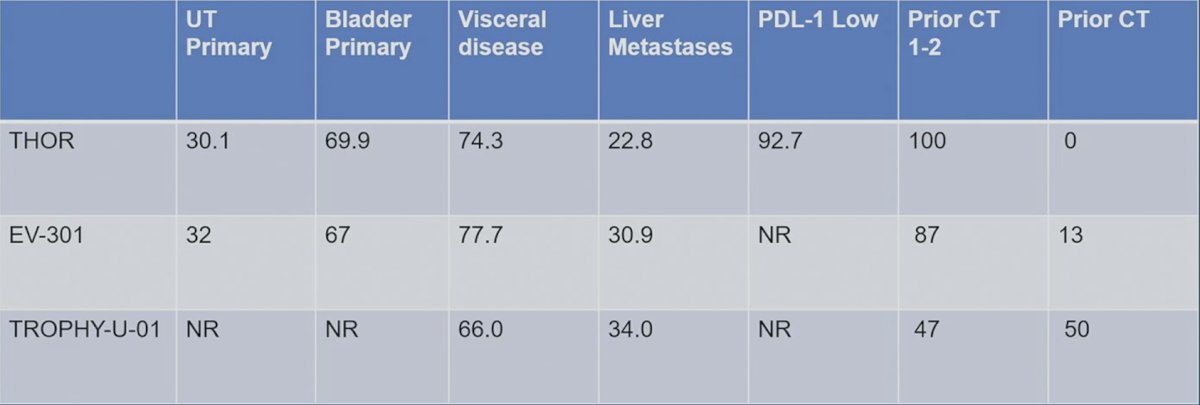
How do we select third line of therapy for metastatic urothelial carcinoma? Primarily by assessing efficacy and toxicity from the available treatment options. As follows is a comparison summary of the patient characteristics for THOR, EV-301, and TROPHY-U-1:
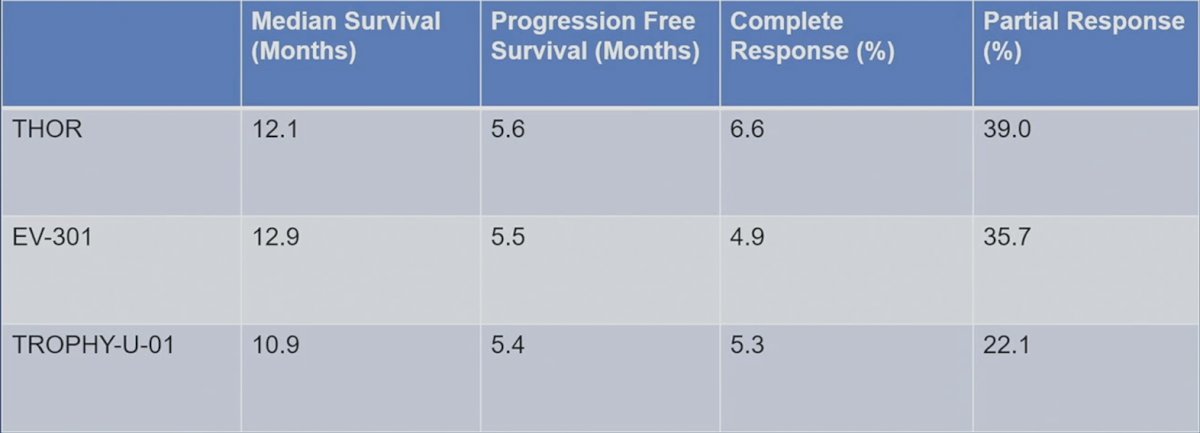
The efficacy results between these 3 trials are quite comparable, with median overall survival ranging from 10.9-12.9 months, progression free survival ranging from 5.4-5.6 months, complete response rates 4.9-6.6%, and partial response rates 22.1-39.0%:
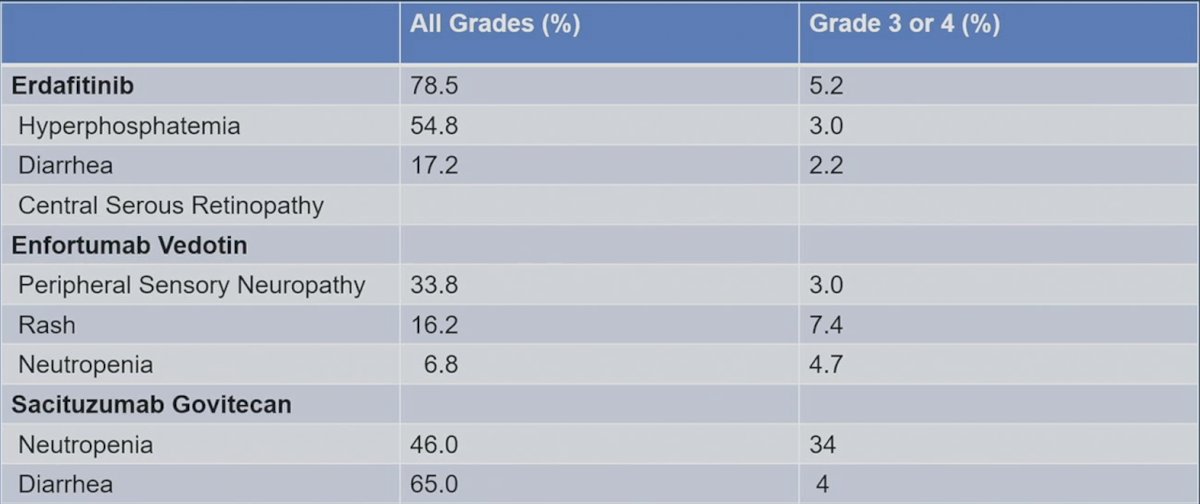
When assessing toxicity, there are some nuances, notably 34% grade 3-4 neutropenia and 65% all grade diarrhea for sacituzumab govitecan, and 33.8% all grade peripheral sensory neuropathy for enfortumab vedotin:
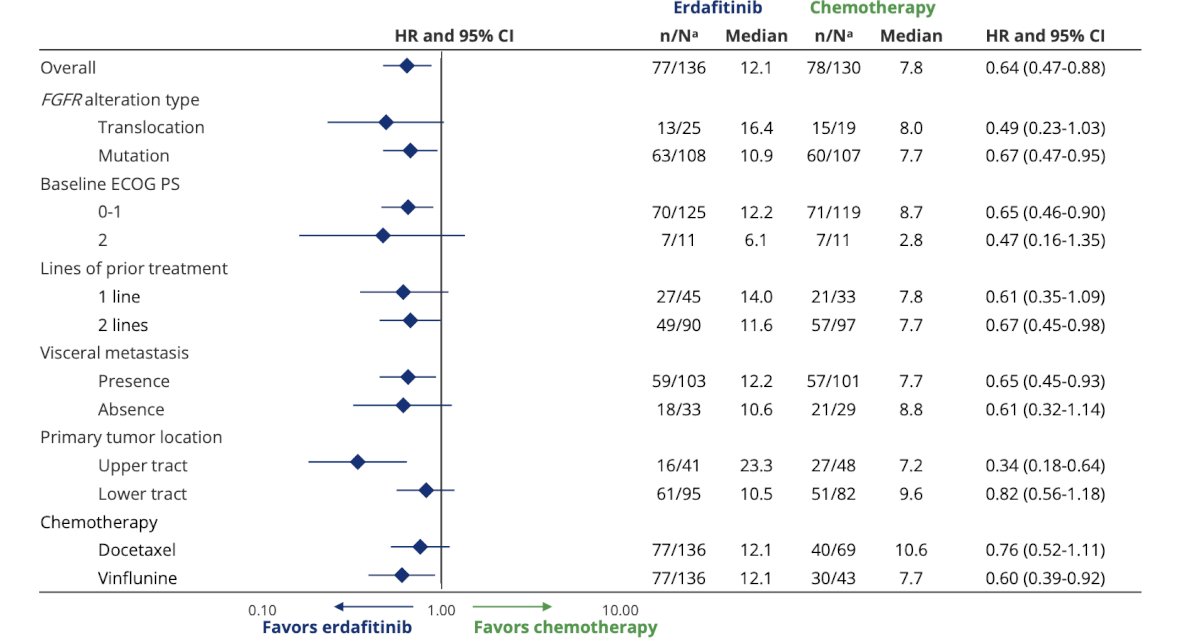
Dr. Petrylak highlighted that enfortumab vedotin has very little activity in FGFR2/3 positive patients, whereas the overall survival benefit with erdafitinib versus chemotherapy was consistently observed across subgroups:
 Future studies should have a focus on resistance mechanisms of FGFR inhibitors. This may include (i) upregulation of alternative pathways (MET, Eph3B, ERBB family), (ii) FGFR gatekeeper mutations, (iii) epithelial to mesenchymal transition, and (iv) activation of intracellular signally pathways (MAPS, PI3K/AKT, JAK/STAT, GSK3B).
Future studies should have a focus on resistance mechanisms of FGFR inhibitors. This may include (i) upregulation of alternative pathways (MET, Eph3B, ERBB family), (ii) FGFR gatekeeper mutations, (iii) epithelial to mesenchymal transition, and (iv) activation of intracellular signally pathways (MAPS, PI3K/AKT, JAK/STAT, GSK3B).
Can inhibition of FGFR2/3 convert a cold to a hot tumor? FGFRalt patients treated on the BLC2001 phase II trial [2] of erdafitinib who previously received checkpoint inhibition had a 5% objective response to the immune checkpoint inhibitor. In vitro studies have demonstrated that treatment with erdafitinib leads to tumor infiltration with CD4+ and CD8+ T cells. Of note, the phase 2 erdafitinib + cetrelimab will be presented later at this meeting.
Dr. Petrylak concluded his discussant presentation of the phase 3 THOR study with the following take-home points:
- All metastatic urothelial patients should be tested for FGFR3/2 alterations, preferably at first diagnosis
- The THOR study demonstrates superior overall survival, progression free survival, and objective response rates of erdafitinib compared to single agent chemotherapy in patients with FGFR3/2 alterations
- Future studies are needed to develop rational sequencing of erdafitinib, enfortumab vedotin, and sacituzumab govitecan
- Understanding the resistance pathways to erdafitinib is essential to developing effective combination therapy, either with immune checkpoint, tyrosine kinase or HDAC inhibitors
Presented by: Daniel P. Petrylak, MD, Yale School of Medicine, New Haven, CT
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Nimgaonkar V, Hubbard RA, Carpenter EL, et al. Biomarker testing, treatment uptake, and survival among patients with urothelial cancer receiving gene-targeted therapy. JAMA Oncol. 2022 Jul 1;8(7):1070-1072.
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2019 Jul 25;381(4):338-348.


