(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a kidney and bladder cancers poster session. Dr. Anupama Reddy presented the results of a study evaluating machine learning models allocating renal cell carcinoma (RCC) patients to treatment arms using biomarker-driven RNA sequencing.
Randomized clinical trials of systemic treatment in the metastatic RCC setting currently randomize patients to treatment arms without considering underlying genomic classifiers of disease biology. RNA sequencing has shown promise in defining the biology of individual RCC tumors. However, these biomarkers have not yet been applied to clinical research or practice for prospectively assigning optimal treatments to patients. Current challenges for RNA-seq biomarker development include:
- Translating classifiers across different assays/platforms
- Normalization of data collected from single patients in the clinic
- Establishing robust thresholds for assigning prediction groups
The phase 2 OPTIC (NCT05361720) RCC study will test the utility of RNA-seq based biomarkers to assign treatment based on biologic drivers relevant to angiogenesis (nivolumab/cabozantinib) and the immune microenvironment (ipilimumab/nivolumab).

In this study, Dr. Reddy and colleagues presented analysis of the development and optimization of a machine learning model for assigning individual patients to biologically driven clusters in real time to facilitate RNA-seq based biomarker trials.
The authors utilized RNA-seq data from the IMmotion 151 trial (atezolizumab + bevacizumab versus sunitinib in patients with metastatic RCC1) to develop a machine learning model. Clusters were grouped into one of three cohorts/signatures based on their underlying tumor biology and treatment response:
- Cluster 1 + 2: Angiogenic signature Receive tyrosine kinase inhibitor (TKI) + immunotherapy (IO)
- Cluster 4 + 5: Immune/proliferative signature Dual IO therapy
- Cluster 3 + 6: Neither signature Excluded


The authors used a random forest classifier to train a multi-class model to predict the three groups, with subsequent validation performed using bootstrapped cross-validation.
The machine learning classifier was developed using 188 genes. The cross-validation accuracy was 85% and the sensitivity >90% for assigning patients into one of the three biologic clusters from the training data.
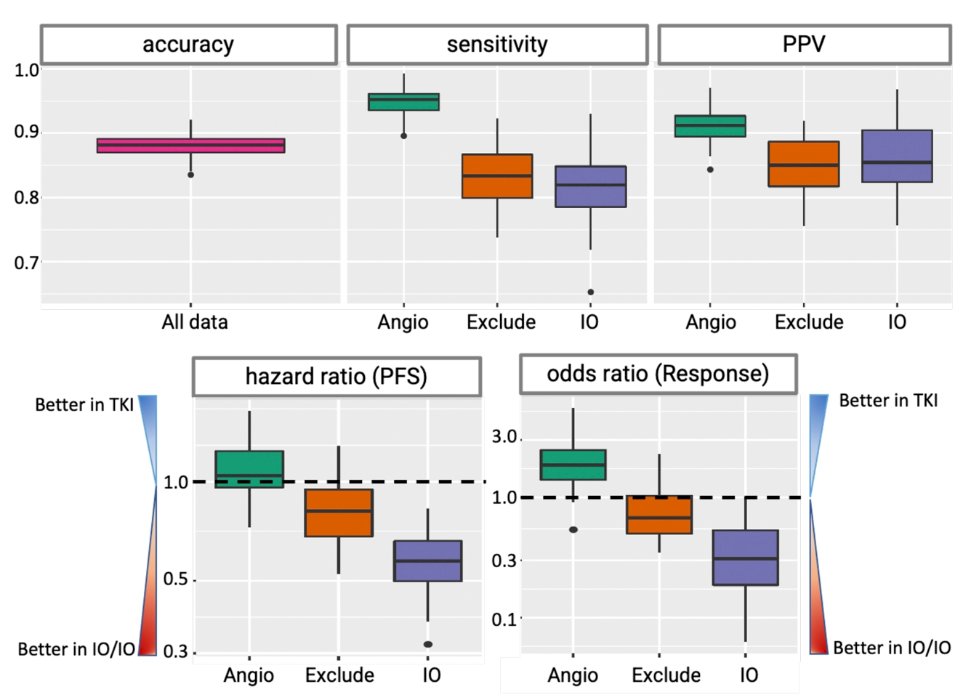
The predictions of the classifier (i.e., the assigned genomic classifier signature) were significantly associated with progression-free survival across the different treatments, within each of the predicted groups.
The investigators also observed significant odds ratios when comparing responders (CR/PR/SD) to non-responders (PD) across the treatment groups.
The model was then validated in two independent test sets treated with TKIs and IO therapy:
- 61 patient RCC cohort (all subtypes)
- 12 patient clear cell RCC cohort
Within the angiogenic cluster signature (cluster 1+2), patients receiving combination TKI+IO had significantly better responses compared to patients receiving dual IO therapy (p=0.05).

Dr. Reddy and colleagues concluded that they have developed an accurate machine learning model to assign individual patients to RNA-seq clusters in real time. This classifier will facilitate the prospective OPTIC RCC trial, and, If successful, this biomarker strategy will serve as a proof of concept for selecting optimal treatments for RCC patients.

Presented by: Anupama Reddy, PhD, Data Scientist, Vindhya Data Science, Raleigh-Durham, NC
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomized controlled trial. Lancet 2019 Jun 15;393(10189):2404-2415.


