(UroToday.com) The 2023 ASCO annual meeting included a kidney cancer session, featuring a presentation by Dr. Daniel Heng discussing real-world outcomes among patients with advanced/metastatic RCC treated with cabozantinib or other TKIs after checkpoint inhibitor therapy. Following disease progression on or after first-line checkpoint inhibitor therapy, global clinical guidelines currently recommend VEGF TKIs as second line therapy for patients with metastatic RCC. However, there are limited clinical data on the effectiveness of VEGF-targeted therapies in this setting, particularly for cabozantinib, which demonstrated superiority over everolimus after VEGF-targeted therapy in the phase 3 METEOR trial.1 In the US, cabozantinib is approved for patients with metastatic RCC with no restrictions on prior therapy, however, in Europe and Canada, cabozantinib monotherapy is only approved after VEGF-targeted therapy.
This was a retrospective study of cabozantinib vs other TKI treatments after cabozantinib checkpoint inhibitor therapy for mRCC using the US Oncology Network electronic health record database and chart review. Patients initiated TKI therapy between May 1, 2016, and Nov 30, 2021, immediately after checkpoint inhibitor therapy. The primary endpoint was real-world response rate over the first 6 months of treatment based on physician assessment. Complete response was defined as documented complete response/indication of remission/disappearance of all lesions/no evidence of disease. Partial response was defined as documented partial response/improved disease/responding disease. Covariates were adjusted by inverse probability of treatment weighting. Noninferiority was assessed using a 90% CI and 10% noninferiority margin; if noninferiority was met, superiority was evaluated using a 95% CI and chi-square test.
Overall, 485 patients were included in this study:

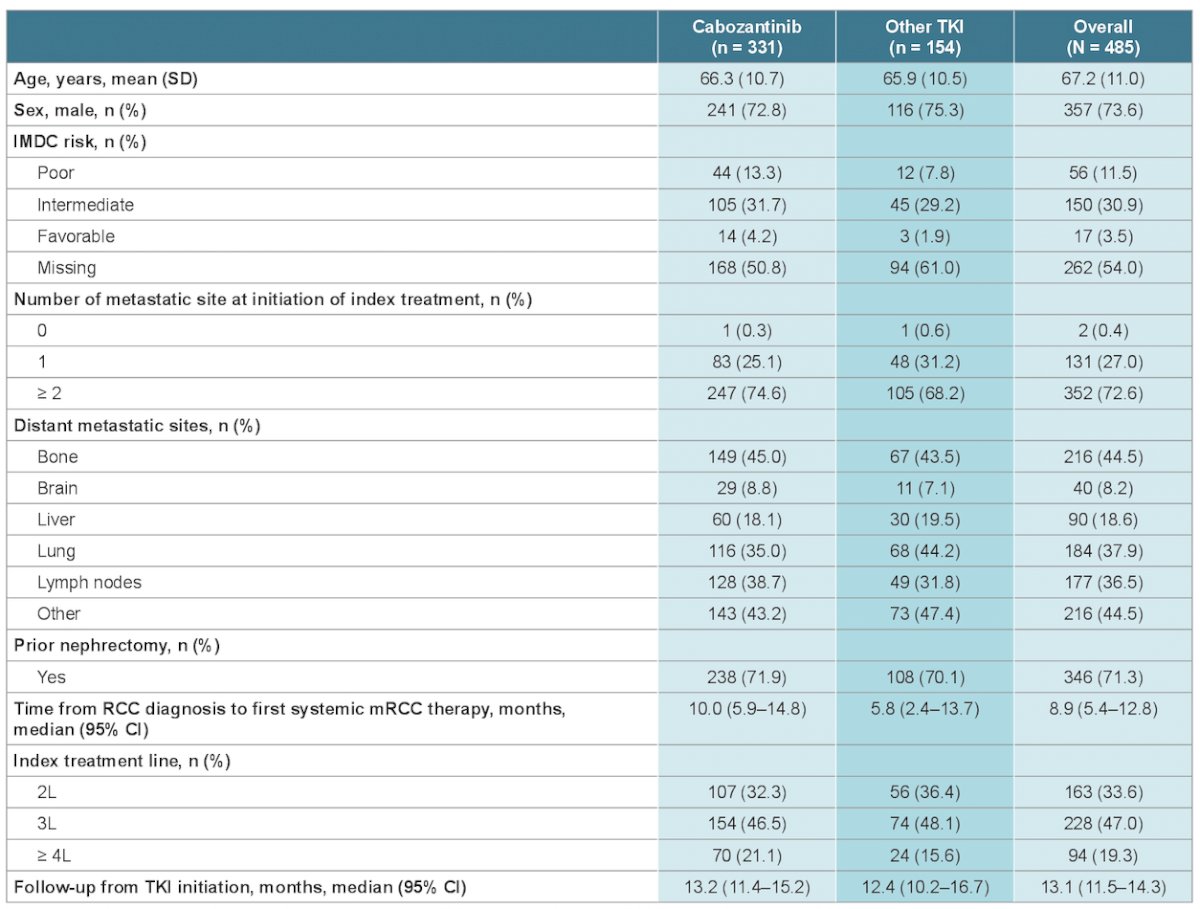
Baseline characteristics were similar for the cabozantinib and other TKIs subgroups, including proportions of patients with ≥ 2 metastatic sites (74.7% vs 68.2%) and metastases in bone (45.0% vs 43.5%), brain (8.8% vs 7.1%), liver (18.1% vs 19.5%), lung (35.0% vs 44.2%) and lymph nodes (38.7% vs 31.8%). Baseline characteristics are summarized in the following table:
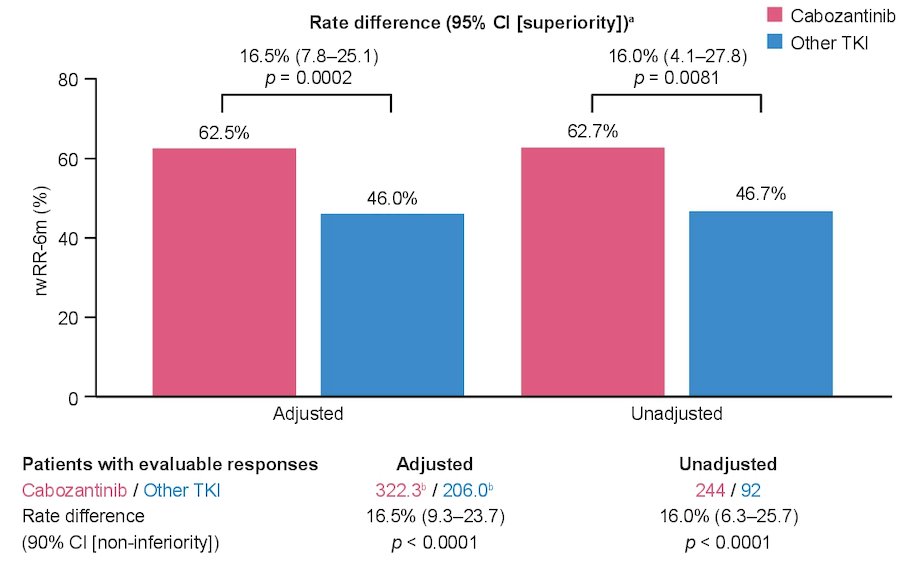
Over the first six months of index treatment, 16.5% of patients died without any tumor assessment (13.6% cabozantinib arm, 22.7% other TKI arm). At 6 months, tumor assessment data were available for 75.5% of patients. Adjusted real-world response rate over the first 6 months of treatment was 62.5% for cabozantinib and 46.0% for other TKIs (rate difference: noninferiority, 16.0% [90% CI, 6.3–25.7], p < 0.0001; superiority, 16.0% [95% CI, 4.1–27.8], p = 0.0081):
Finally, there was no difference in OS, however, there was a numerical improvement in time to next treatment for cabozantinib versus other TKIs. These results were consistent between IPTW-adjusted and unadjusted analyses:

Dr. Heng concluded his presentation by discussing real-world outcomes among patients with advanced/metastatic RCC treated with cabozantinib or other TKIs after checkpoint inhibitor therapy with the following take-home messages:
- In patients with mRCC receiving standard of care treatment in the USA, cabozantinib was effective post-checkpoint inhibitor therapy, including in patients without prior TKI therapy
- Time to next treatment was numerically higher with cabozantinib than with other TKIs, while OS was similar between treatment arms, regardless of adjustment
- These data may inform global jurisdictions that restrict cabozantinib to the post TKI setting
Presented by: Daniel Y. C. Heng, MD, MPH, University of Calgary, Calgary, AB, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373(19):1814-1823.


