(UroToday.com) Dr. Manuela Schmidinger provides a nice review and interpretation of three abstracts focused on Non-clear cell renal cell carcinoma (nccRCC). Her talk was entitled “Adding Clarity to Non-Clear Cell Carcinoma.”
The three abstracts she reviewed today were the following:
- Abstract 4518: First-line lenvatinib + pembrolizumab treatment across non-clear cell renal cell carcinomas: Results of the phase 2 KEYNOTE-B61 study
- Abstract 4519: Efficacy of first-line (1L) immunotherapy (IO)-based regimens in patients with sarcomatoid and/or rhabdoid (S/R) metastatic non-clear cell renal cell carcinoma (nccRCC): Results from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC)
- Abstract 4520: Phase II study of cabozantinib (Cabo) with nivolumab (Nivo) and ipilimumab (Ipi) in advanced renal cell carcinoma with variant histologies (RCCvh)
All three abstracts were summarized and covered by Urotoday and can be found separately.
She began by highlighting, as all three authors had, that the treatment of nccRCC remains a challenge in 2023. She offered the following reasons:
- Guidelines offer weak recommendations (IIa-c) and include most TKI’s that were used primarily for ccRCC
- Many basket trials that had small variant histology (VH) subgroups, underreporting of subgroup outcomes, mixed populations in later settings, and significant intra-variant heterogeneity
- Outcomes with TKI’s are often overestimated as they liked to perform better for ccRCC than nccRCC. Realistically, ORR with TKIs is likely around 4-28% and PFS around 8 months
- There have been many new developments, including improved outcomes (ORR 23-72%, PFS up to 21 months), MET-inhibitors for MET driven tumors, IO-targeted therapy combos, etc. These have not been clearly delineated for nccRCC specifically.
She notes that basket trials may have their merits, because it may allow a faster way to new benchmarks – as long as nccRCC subgroup analyses are planned.
First, she focused on Abstract 4518. Dr. Lee presented the results of the phase 2 KEYNOTE-B61 study, which evaluated first line lenvatinib + pembrolizumab treatment across non-clear cell renal cell carcinomas. Initial results of a phase II single-arm KEYNOTE-B61 trial have previously been reported and have demonstrated anti-tumor activity of this combination in patients advanced non-clear cell RCC. Summary of that data can be found here: https://www.urotoday.com/video-lectures/esmo-2022/video/2828-esmo-2022-keynote-b61-laurence-albiges.html. Objective response rate was 47.6% and disease control rate was 79%.
The original study design is seen below: 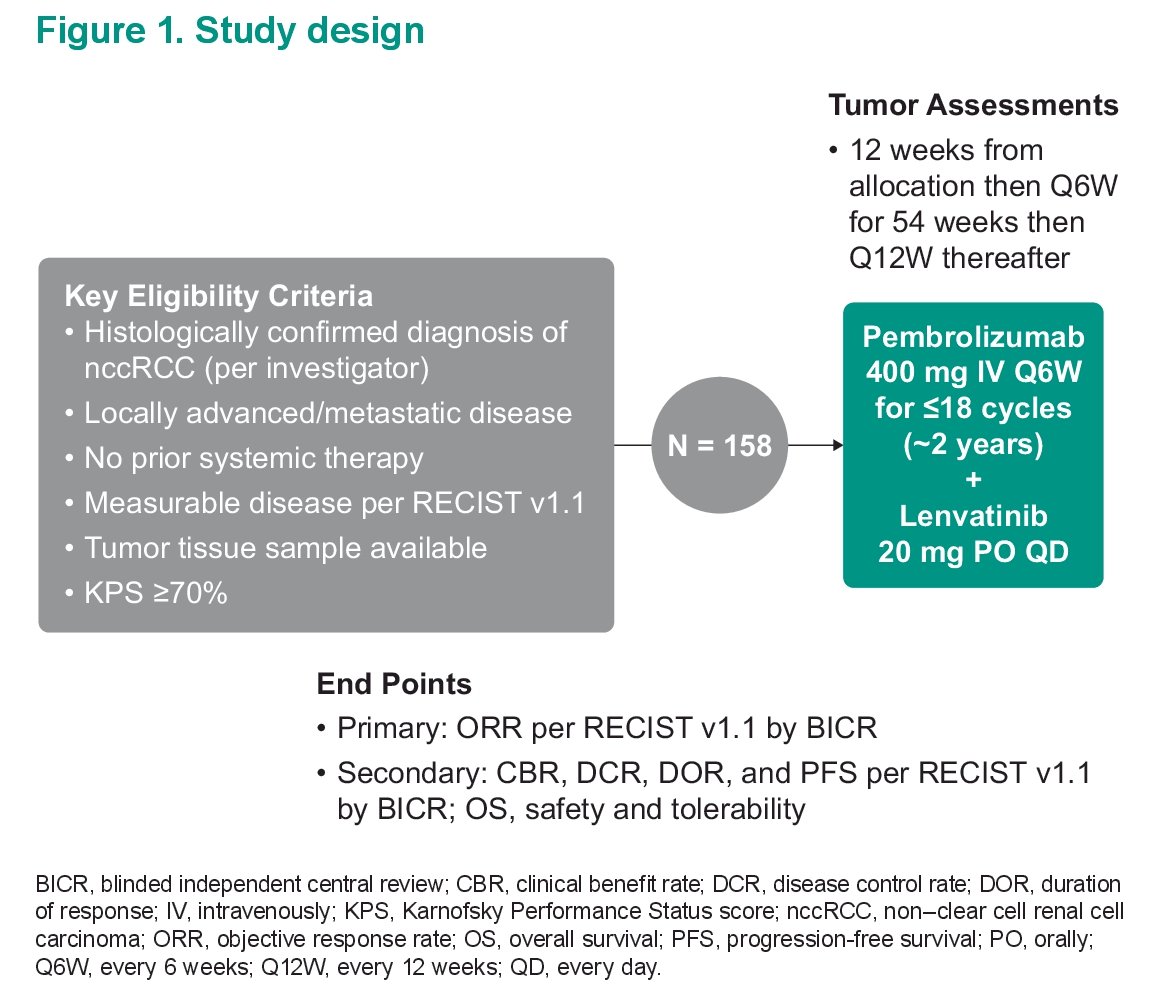
This year’s ASCO abstract presented updated data with median 14.9 months follow-up.
She notes that in this study, most patients had papillary RCC (59%) or chromophobe RCC (19%). 44% had IMDC favorable risk disease, which is enriched – she feels this may contribute to the results below.
Looking at updated outcomes: 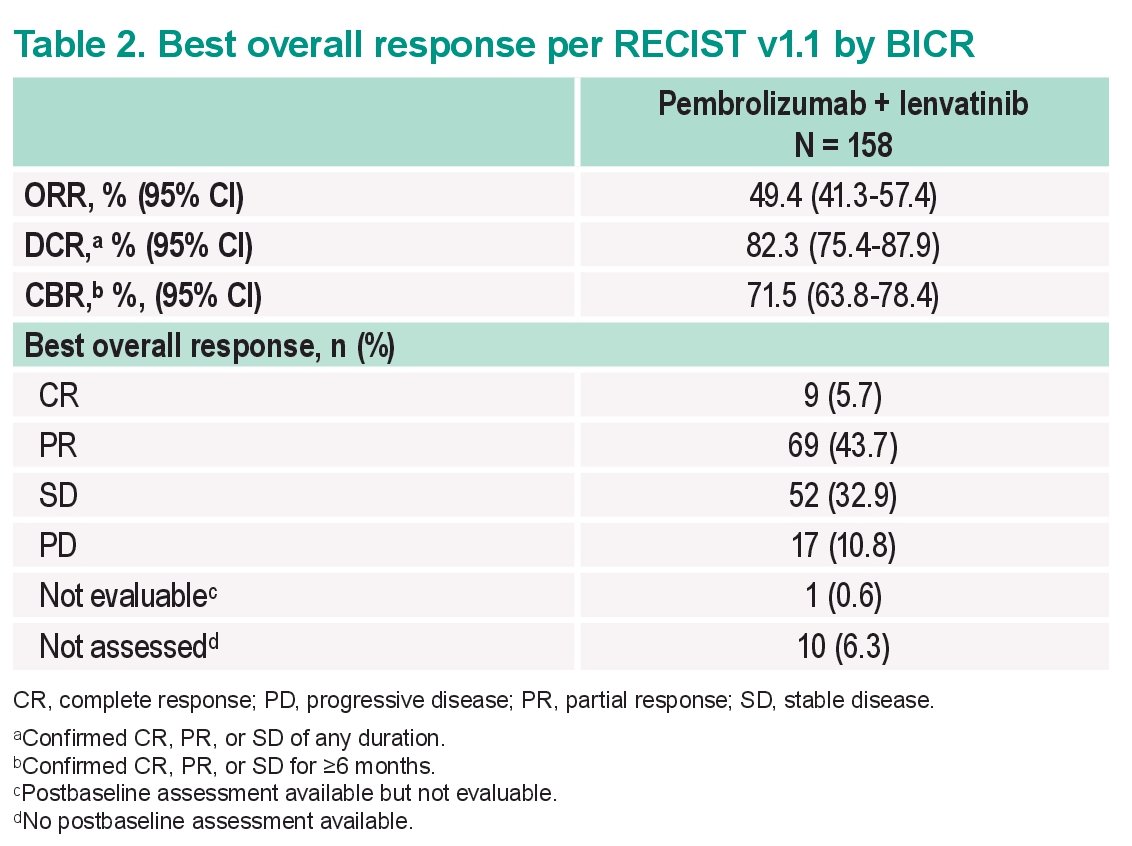
- ORR remains high at 49%
- DCR remained high at 82%
- CR rates were 6%
Of note, median duration of response was not reached. 75% of responders remained in response >= 12 months, which is encouraging. This translated into a very important PFS signal – with a median PFS of 17.9 months and a median OS that has not been reached. Generally, toxicity was in line with previously reported.
Dr. Schmidinger then noted that this author (Dr. Lee) has a dilemma on their hands – because while the LenPem data is promising, they also published data on the combination of nivolumab/cabozantinib. That trial’s updated data (excluding chromophobe RCC) was also presented – and while head-to-head comparisons aren’t ideal, it is worth looking at.
Below is a comparison of the non-chromophobe RCC populations: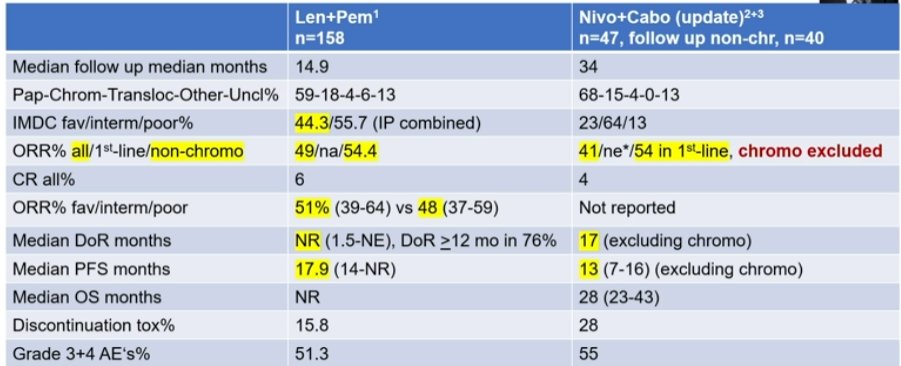
They are remarkably similar, with a slightly higher PFS for LenPem – but their population is enriched for favorable risk IMDC patients.
So, her take-away is the following:
- Is LenPem practice changing? A new benchmark is certainly set, but the nivo/cabo data similarly promising
- Impact on patient care? We should probably go for IO-TKI combination in nccRCC despite the lack of Phase 3 trial – at least in non-chromophobe patients.
- Uncertainties remain:
- Is the high number of favorable risk IMDC patients limiting? She notes the ORR was similar for intermediate/poor risk – but we should await PFS data
- What is the real contribution of pembro in chromophobe? CD-8 infiltrates in chRCC have a predominantly non-exhausted immune phenotype, lack of antitumor activity
- Is efficacy in chromophobe primarily driven by Lenvatinib? Perhaps this provides rationale for VEGF-FGF-TKI in chromophobe.
She then moved on to Abstract 4519. In this abstract, Dr. Labaki reported on the efficacy first-line (1L) immunotherapy (IO)-based regimens in patients with sarcomatoid and/or rhabdoid (S/R) metastatic non-clear cell renal cell carcinoma (nccRCC). It is work from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) was utilized to identify patients with metastatic nccRCC (both S/R and non S/R) who are treated in the first line with either IO based regimens (IO/IO or IO/VEGF) or VEGF monotherapy. their goal was primarily to assess overall survival and time to treatment failure but they also looked at overall response rate as a secondary outcome. They did account for age, IMDC risk group, and nccRCC histologic subtype.
She notes that in the S/R cohort of patients, most patients had intermediate or poor IMDC risk disease and 70% received dual IO therapy combinations. In contrast, in the non S/R nccRCC group, more patients were favorable risk patients and less received IO based regimens.
Looking first at OS:
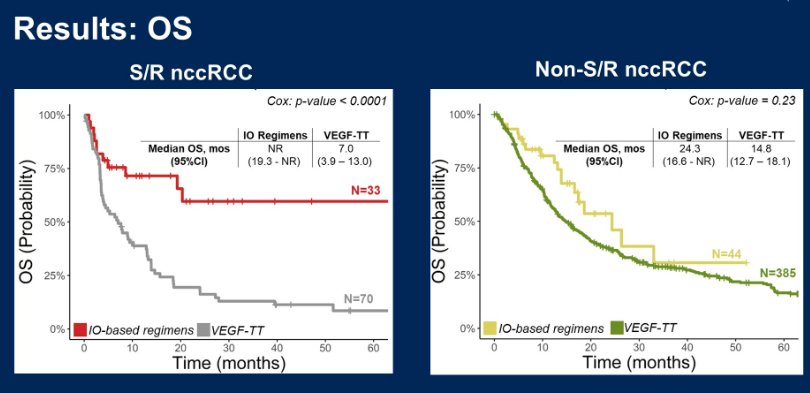
She notes that they reported a significant risk reduction for death (75%) as seen on the figure on the left, with the use of IO based regimens for S/R nccRCC patients. They also had a 66% risk reduction in treatment failure in that population. However, these same benefits were NOT seen in the non S/R nccRCC patients – but she said to bear in mind that this population had a large favorable risk cohort and 57% still received IO doublet therapy, which may not have been the best choice of treatment.
Her key take-aways from this abstract:
- This is the largest series of patients with nccRCC with S/R differentiation
- Similar to sarcomatoid ccRCC, IO based regimens outperform targeted therapies in S/R nccRCC
- 75% risk reduction in death! (HR 0.25)
- This should be practice changing.
Lastly, she delved into Abstrast 4520. Dr. McGregor presented the results of a phase II study of cabozantinib (Cabo) with nivolumab (Nivo) and ipilimumab (Ipi) in advanced renal cell carcinoma with variant histologies (RCCvh).
The study schema is seen below:
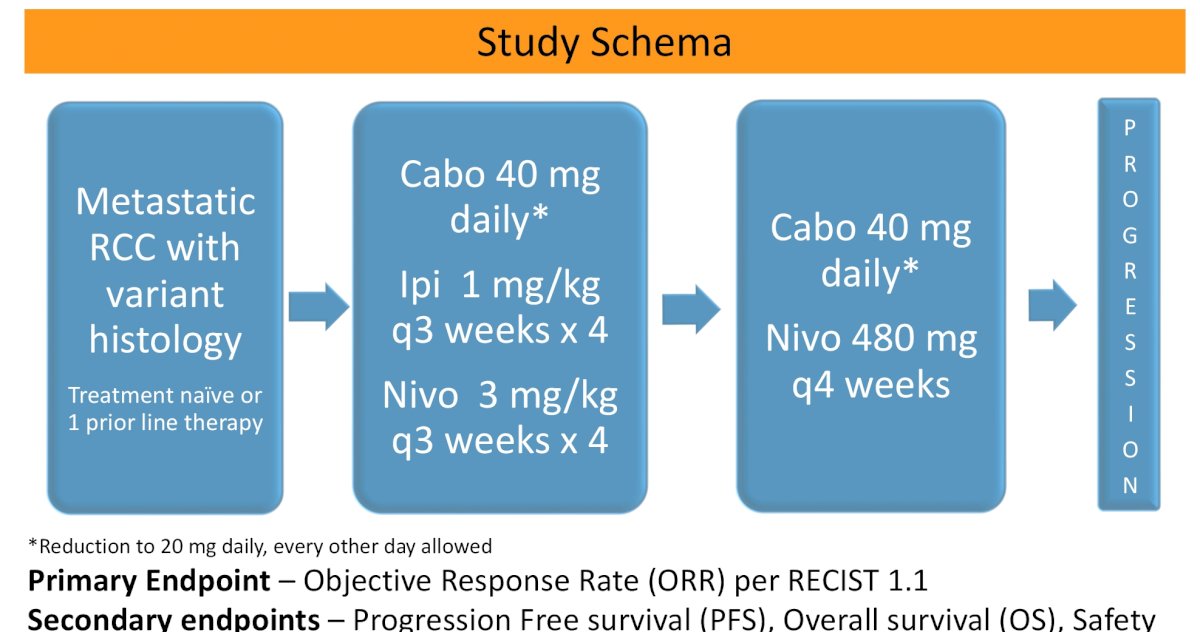
Median follow-up was short at 10.4 months. Most patients had intermediate/poor risk disease, 51% had papillary RCC, 28% chromophobe RCC, and 23% had sarcomatoid features.
She notes that this was not a well tolerated combination - Looking at treatment exposure in these 39 patients, 44% received all four doses of Nevada and EPI while 34% receive two doses or less. 90% of patients had dose reduction in cabozantinib.
An objective response was seen in eight patients which results in an objective response rate of 21%. ORR broken down below:
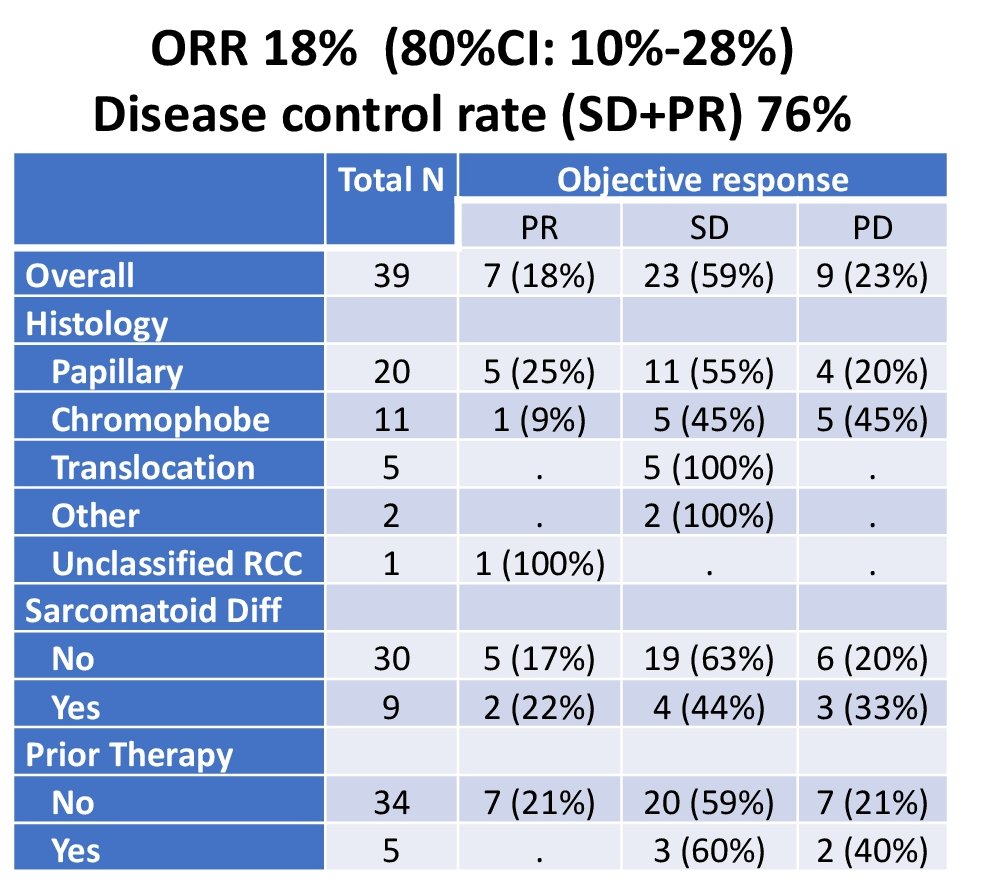
- ORR was best in papillary RCC and less in chromophobe RCC.
Unfortunately, as noted by the dose reductions, TRAEs were high. 74% developed treatment-related grade 3 or higher toxicities. This included 37% (n=14) developed ≥ grade 3 elevation in AST or ALT. 29% (n=11) required high dose steroids (prednisone ≥ 40 mg daily or equivalent).
She also noted the response rates relative to ccRCC followed the trend of prior targeted therapies – significantly lower in the variant histology cohort compared to ccRCC. The data on the left is from the COSMIC trial data (ccRCC) vs. the current phase II trial data on the right.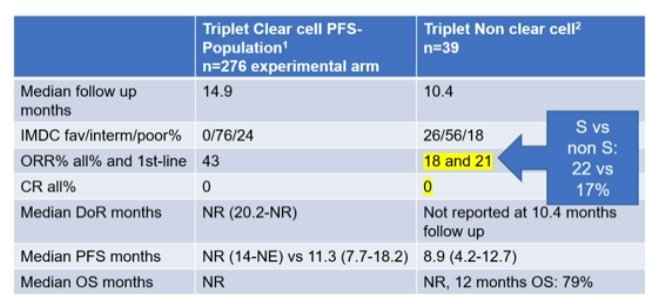
Key take-aways from the Triplet-Therapy for nccRCC:
- CaNi study safety: Similar to clear cell RCC, safety and tolerability remains a concern. Triplet therapy may be being given at the expense of nivolumab maintenance and appropriate cabozantinib dosage
- CaNi study efficacy – ORR and PFS look modest, but follow-up is too short to estimate a potential benefit from the IPI component
- CaNi study – should we even put patients onto this study? Yes, she feels it is still worth considering as it remains important to study the role of IPI in this nccRCC cohort.
With that, she concluded. This was a great assessment of 3 abstracts focused on the nccRCC disease space.
Presented by: Manuela Schmidinger, MD | Medical University of Vienna
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis, @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


