(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a kidney and bladder cancers poster session. Dr. David McDermott presented the rationale and study framework for LITESPARK-024, a randomized phase 1/2 trial of belzutifan +/- palbociclib for the treatment of advanced renal cell carcinoma (RCC) patients.
Numerous combinations are currently approved in the first-line treatment setting for patients with advanced RCC. These regimens combine immune checkpoint inhibitors (ICI) together (e.g., ipilimumab + nivolumab) or with an anti-angiogenic agent, such as a vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKI), such as pembrolizumab + axitinib. Despite promising results in the 1st line setting,1,2 many patients develop subsequent treatment resistance, and effective second- and third-line treatment options are needed.
Belzutifan, a hypoxia-inducible factor 2α (HIF-2α) transcription factor inhibitor, has demonstrated promising clinical efficacy in patients with previously treated advanced clear cell RCC in a phase 1/2 trial, whereby patients receiving oral belzutifan 120 mg once daily had an objective response rate of 25%, with a median progression-free survival of 14.5 months.3
The cyclin-dependent kinase (CDK) pathway is similarly dysregulated in patients with RCC. Palbociclib, a CDK4/6 inhibitor has previously demonstrated inhibitory effects in RCC cell lines. It has been suggested that simultaneous CKD 4/6 and HIF-2α inhibition may have synergistic mechanisms of action, and thus there has been an increased interest in evaluating such agents in tandem. LITESPARK-024 (NCT05468697) is an open-label, multicenter, randomized, phase 1/2 trial evaluating the combination of belzutifan plus palbociclib versus belzutifan alone in patients with advanced clear cell RCC who had disease progression following prior anti-PD-1/L1 immunotherapy and a VEGF-TKI, either sequentially or in combination.
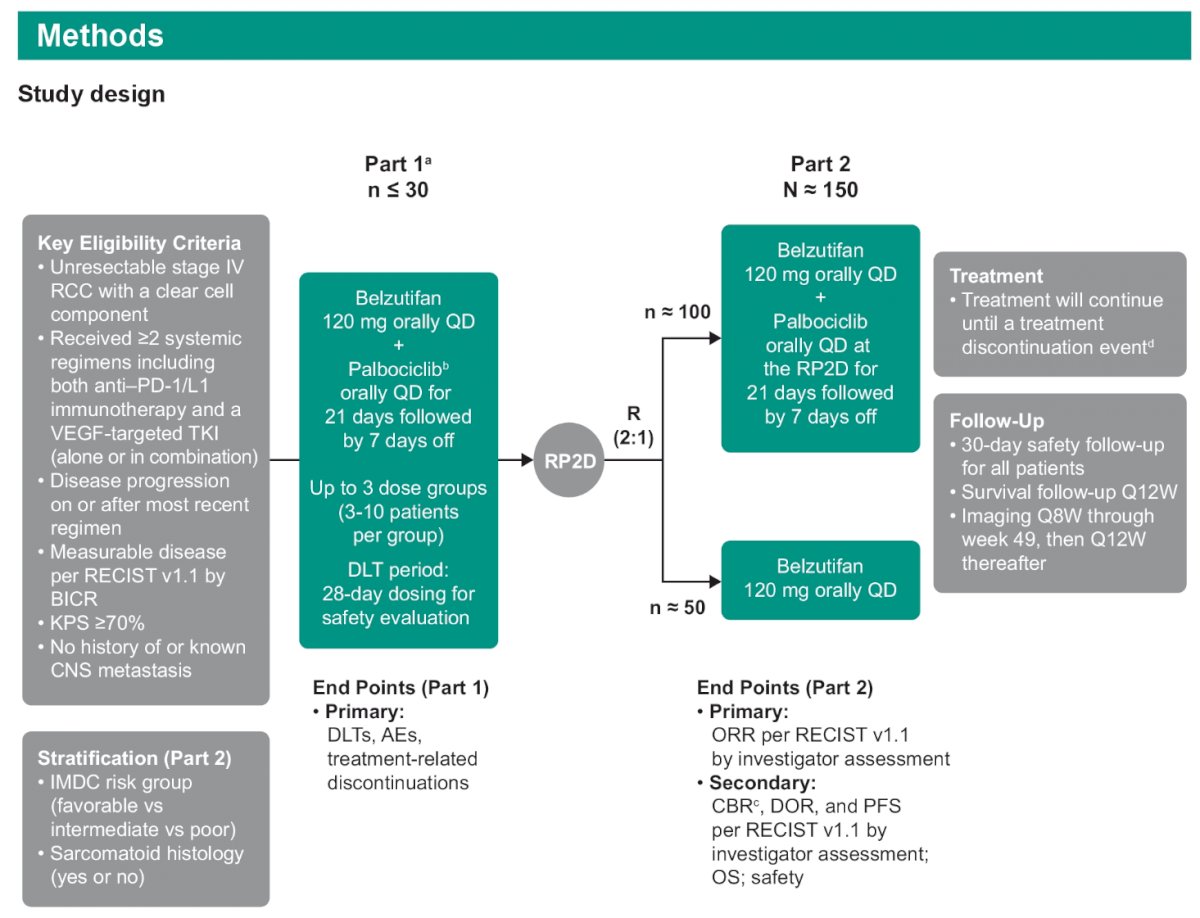
This trial will consist of two parts:
- Part 1 (n ≤30):
- Patients will receive belzutifan 120 mg orally daily plus palbociclib orally daily for 21 days followed by 7 days off. The palbociclib dose will be sequentially increased starting with 75 mg, increasing to 100 mg, and then 125 mg, depending on any dose-limiting toxicities (DLTs) observed.
- Objectives: Assess DLTs, adverse events (AEs), treatment-related discontinuations
- Part 2 (n=150):
- After the recommended phase 2 dose (RP2D) is determined, patients will be randomized 2:1 to either belzutifan + palbociclib at the RP2D or belzutifan alone.
- Objectives: Assess objective response rate (ORR) per RECIST v1.1 (investigator-assessed), clinical benefit rate, duration of response, progression-free survival (PFS), per RECIST v1.1 (investigator-assessed), overall survival (OS), and safety
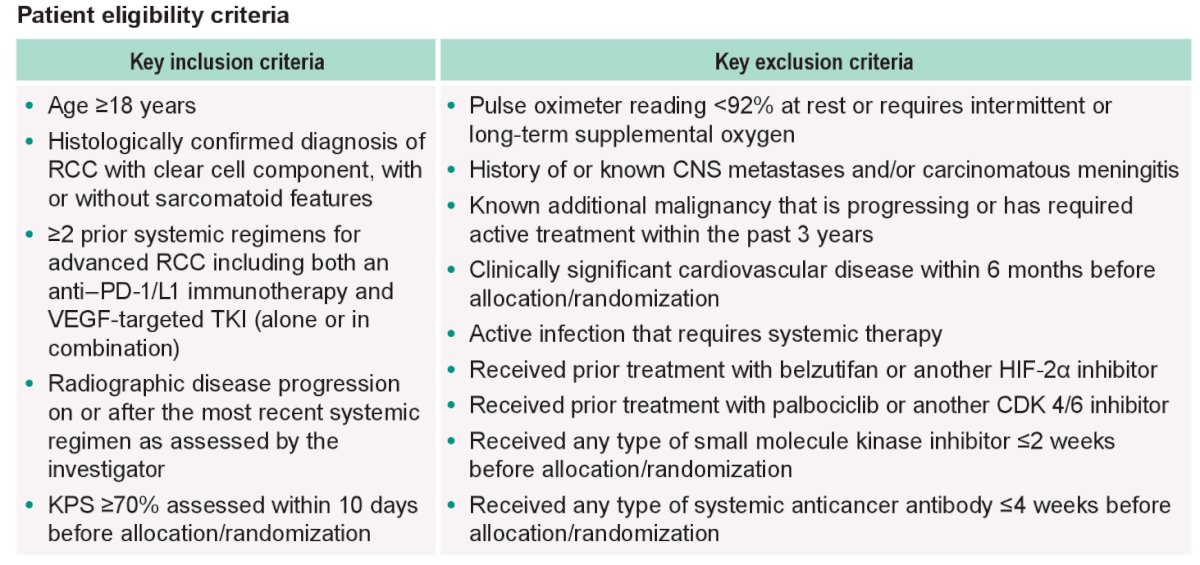
Assessment and patient follow-up is as detailed below:
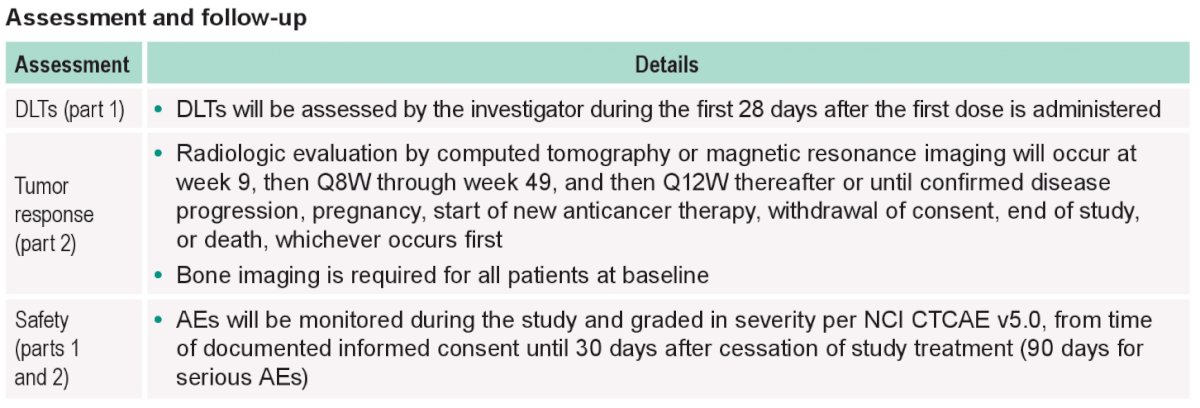
Safety analyses will be performed for all allocated patients who received at least one dose of study treatment. Duration of response will be assessed in partial and complete responders and will be estimated using Kaplan Meier curves. OS and PFS will be estimated using Kaplan Meier curves as well, with the magnitude of treatment difference (i.e., hazard ratios and 95% confidence intervals) assessed using a stratified Cox proportional hazards model with the Efron method of tie handling.
The study has begun enrollment as of July 2022 in Australia, United States, Israel, with additional countries in Europe and Latin America planned for enrollment in the future as well.
Presented by: David F. McDermott, MD, Professor, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.References:
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1116-1127.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carinoma. N Engl J Med 2018;378(14):1277-1290.
- Choueiri TK, Bauer TM, Papadopoulos KP, et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med 2021;27(5):802-805.


