(UroToday.com) The 2023 ASCO annual meeting included a kidney cancer session, featuring a presentation by Dr. Brian Rini discussing the 5-year analysis of KEYNOTE-426, assessing pembrolizumab + axitinib versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (RCC).
At the first interim analysis (median follow-up 12.8 months) of the randomized, open-label, phase 3 KEYNOTE-426 (NCT02853331) study, first-line pembrolizumab + axitinib showed statistically significant overall survival (HR 0.53, 95% CI 0.38-0.74), progression free survival (HR 0.69, 95% CI 0.57-0.84), and objective response rates (59.3% vs 35.7%) over sunitinib for advanced clear cell RCC.1 Continued extended benefit with pembrolizumab + axitinib vs sunitinib was observed with extended follow-up (median follow-up, 30 months), overall survival HR 0.68 [2]. And in the protocol-specified final analysis (median follow-up 42.8 months), overall survival HR 0.73, there was continued benefit. At the ASCO 2023 annual meeting, Dr. Rini and colleagues report results with 5-year minimum follow-up and median follow-up of 67 months.
Adults with confirmed locally advanced or metastatic clear cell RCC with or without sarcomatoid features, no previous systemic therapy for metastatic clear cell RCC, KPS ≥70%, and ≥1 lesion measurable per RECIST v1.1 were randomly assigned 1:1 to receive pembrolizumab 200 mg IV Q3W for 35 doses (~2 years) + axitinib 5 mg PO BID or sunitinib 50 mg PO QD on a 4-week-on/2-week-off schedule. The trial design for KEYNOTE-426 was as follows:
Dual primary end points were overall survival and progression free survival per RECIST v1.1 by blinded independent central review. Secondary end points included objective response rates and duration of response per RECIST v1.1 by blinded independent central review, and safety. A post hoc analysis adjusting for the effect of subsequent therapy on overall survival using a 2-stage adjustment model was conducted. The efficacy was assessed in all patients randomly assigned to treatment (ITT population) and no formal hypothesis testing was performed because statistical significance for overall survival, progression free survival, and objective response rate were met at the first interim analysis.
Among the 861 enrolled patients, 432 were assigned to pembrolizumab + axitinib and 429 to sunitinib. The median study follow-up was 67.2 months (range, 60.0-75.0). The current patient disposition is as follows, notably with 7.0% of patients randomized to pembrolizumab + axitinib still receiving therapy, and 4.5% randomized to sunitinib still receiving therapy: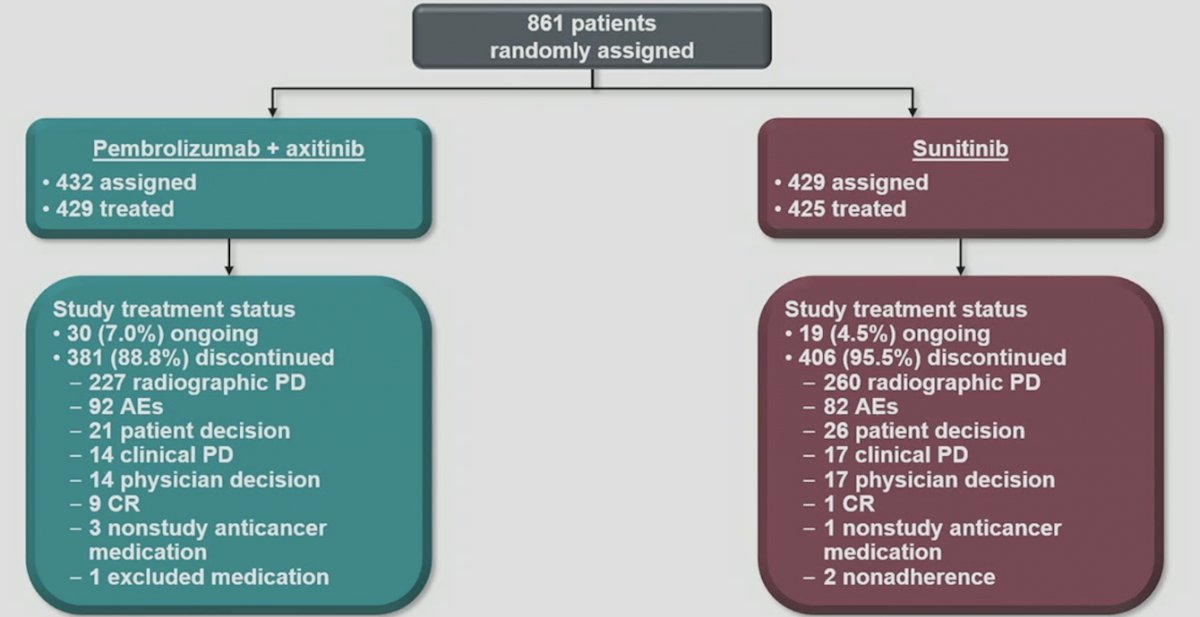
Progression free survival in the ITT population continued to favor pembrolizumab + axitinib vs sunitinib: HR 0.69, 95% CI 0.59-0.81; the 60-month progression free survival rates were 18.3% vs 7.3%: 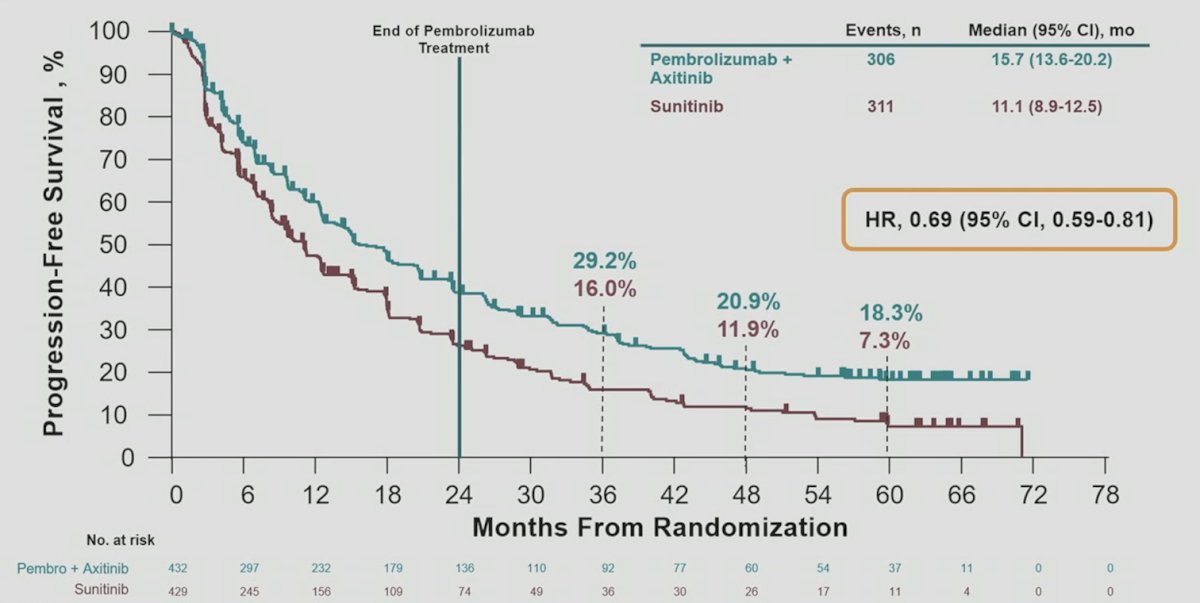
The objective response rate was 60.6% for pembrolizumab + axitinib compared to 39.6% for sunitinib: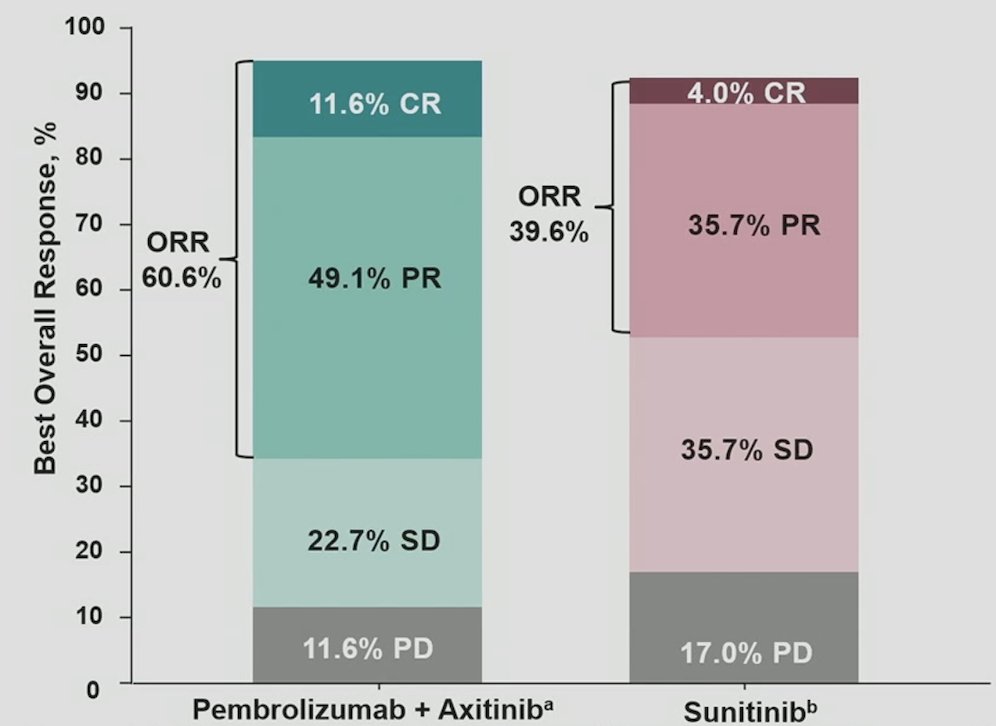
Median duration of response was 23.6 months (range: 1.4+ to 68.6+) for pembrolizumab + axitinib and 15.3 months (range 2.3-68.3) for sunitinib: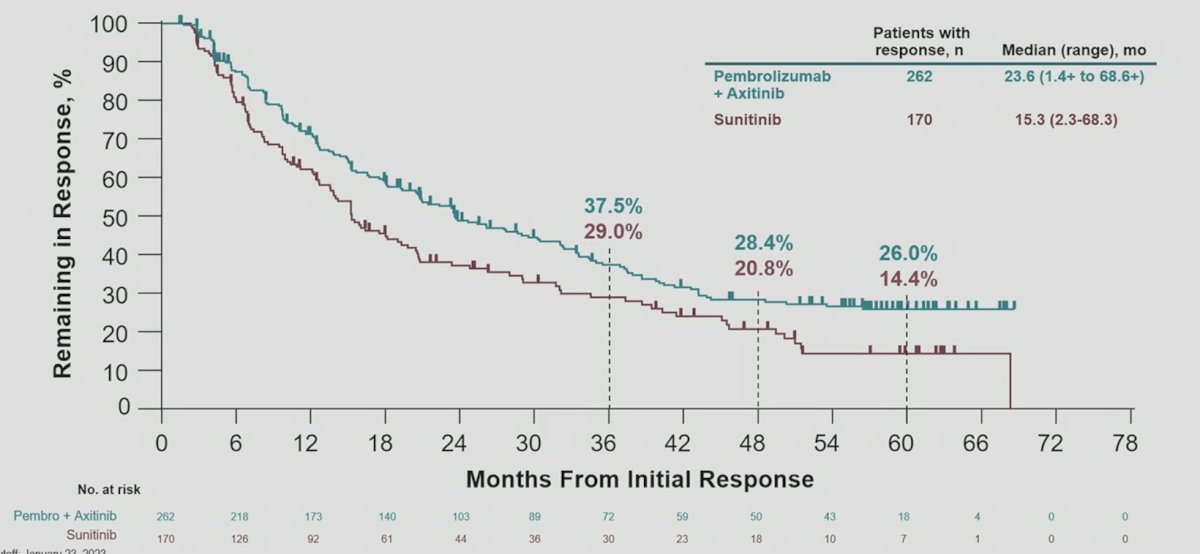
Overall survival in the ITT population continued to favor pembrolizumab + axitinib vs sunitinib: HR 0.84, 95% CI 0.71-0.99; the 60-month overall survival rates were 41.9% vs 37.1%: 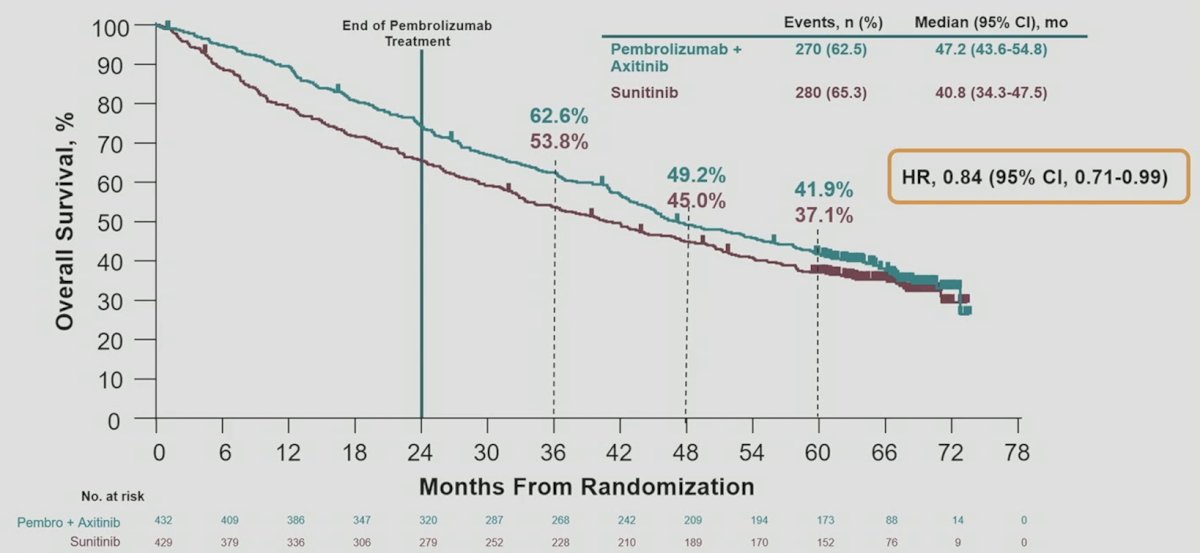
Among patients with IMDC favorable risk disease, there was no difference in overall survival (HR 1.10, 95% CI 0.79-1.54) nor progression free survival (HR 0.76, 95% CI 0.57-1.02) for pembrolizumab + axitinib vs sunitinib, with a slight benefit for pembrolizumab + axitinib (68.8%) versus sunitinib (50.4%) with regards to objective response rate. Contrarily, among patients with IMDC intermediate/poor risk disease, there was a significant benefit in overall survival (HR 0.76, 95% CI 0.62-0.93) and progression free survival (HR 0.68, 95% CI 0.56-0.82) for pembrolizumab + axitinib vs sunitinib, with an additional benefit for pembrolizumab + axitinib (56.8%) versus sunitinib (34.9%) with regards to objective response rate:
In patients who discontinued treatment, 237/381 patients (62.2%) in the pembrolizumab + axitinib arm and 300/406 patients (73.9%) in the sunitinib arm received subsequent anticancer treatment, most commonly VEGF/VEGFR inhibitor for both arms (86.9% vs 72.0%). Notably, the hazard ratio for overall survival when adjusted for subsequent therapy was 0.67 (95% CI 0.52-0.84): 
Additionally, there were 120 patients (27.8%) of patients that completed 35 cycles of pembrolizumab, with the following characteristics: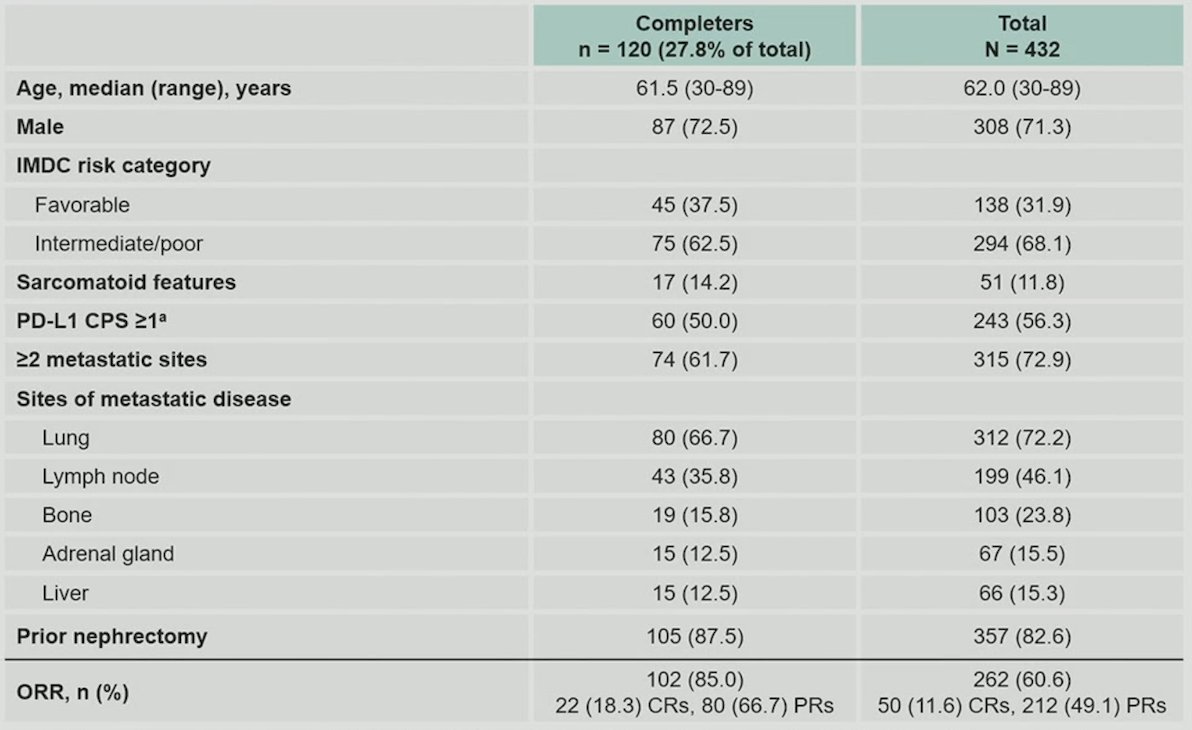
Among these patients, the median overall survival has not been reached (95% CI 70.6-not reached), the 36 month overall survival rate is 94.2%, and the 60 month overall survival rate is 70.7%. With regards to progression free survival, the median progression free survival is 37.4 months (95% CI 32.3-43.7), the 36 month progression free survival rate is 55.2% and the 60 month progression free survival rate is 32.8%.
No new safety signals were observed in this updated analysis.
Dr. Rini concluded his presentation discussing the 5-year analysis of KEYNOTE-426 with the following take-home points:
- KEYNOTE-426 represents the longest follow-up to date of the combination of a checkpoint inhibitor plus a VEGFR/TKI for the first-line clear cell RCC
- Pembrolizumab + axitinib continued to demonstrate improved overall survival, progression free survival, and objective response rate versus sunitinib for patients with previously untreated clear cell RCC
- Benefit was observed despite a greater proportion of patients in the sunitinib arm receiving therapy, including a predominantly PD-1/L1 inhibitors, and more lines of therapy
- A substantial percentage of patients completed 35 cycles of pembrolizumab with good long-term outcomes
- These data continue to support pembrolizumab + axitinib as a standard of care for patients with previously untreated advanced clear cell RCC
Presented by: Brian I. Rini, MD, FASCO, Vanderbilt-Ingram Cancer Center, Nashville, TN
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1116-1127.
- Powles T, Plimack ER, Soulieres D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomized, open-label, phase 3 trial. Lancet Oncol. 2020 Dec;21(12):1563-1573.


